Abstract
Introduction
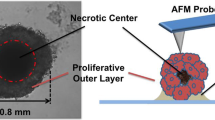
The mechanical interaction between cells and their microenvironment is emerging as an important determinant of cancer progression and sensitivity to treatment, including in ovarian cancer (OvCa). However, current technologies limit mechanical analysis in 3D culture systems. Brillouin Confocal Microscopy is an optical non-contact method to assess the mechanical properties of biological materials. Here, we validate the ability of this technology to assess the mechanical properties of 3D tumor nodules.
Methods
OvCa cells were cultured in 3D using two established methods: (1) overlay cultures on Matrigel; (2) spheroids in ultra-low attachment plates. To alter the mechanical state of these tumors, nodules were immersed in PBS with varying levels of sucrose to induce osmotic stress. Next, nodule mechanical properties were measured by Brillouin microscopy and validated with standard stress–strain tests: Atomic Force Microscopy (AFM) and a parallel plate compression device (Microsquisher). Finally, the nodules were treated with a chemotherapeutic commonly used to manage OvCa, carboplatin, to determine treatment-induced effects on tumor mechanical properties.
Results
Brillouin microscopy allows mechanical analysis with limited penetration depth (~ 92 µm for Matrigel method; ~ 54 µm for low attachment method). Brillouin microscopy metrics displayed the same trends as the corresponding “gold-standard” Young’s moduli measured with stress–strain methods when the osmolality of the medium was increased. Nodules treated with carboplatin showed a decrease in Brillouin frequency shift.
Conclusion
This validation study paves the way to evaluate the mechanics of 3D nodules, with micron-scale three-dimensional resolution and without contact, thus extending the experimental possibilities.





Similar content being viewed by others
References
Ahmed, N., K. Abubaker, J. Findlay, and M. Quinn. Epithelial Mesenchymal Transition and Cancer Stem Cell-Like Phenotypes Facilitate Chemoresistance in Recurrent Ovarian Cancer. Curr. Cancer Drug Targets. 10:268–278, 2010.
Alibert, C., B. Goud, and J. B. Manneville. Are cancer cells really softer than normal cells? Biol Cell. 109:167–189, 2017.
Baker, B. M., and C. S. Chen. Deconstructing the third dimension—how 3D culture microenvironments alter cellular cues. J. Cell Sci. 125:3015–3024, 2012.
Bankhead, C. R., C. Collins, H. Stokes-Lampard, P. Rose, S. Wilson, A. Clements, D. Mant, S. T. Kehoe, and J. Austoker. Identifying symptoms of ovarian cancer: a qualitative and quantitative study. BJOG 115:1008–1014, 2008.
Baraniak, P. R., M. T. Cooke, R. Saeed, M. A. Kinney, K. M. Fridley, and T. C. McDevitt. Stiffening of human mesenchymal stem cell spheroid microenvironments induced by incorporation of gelatin microparticles. J. Mech. Behav. Biomed. Mater. 11:63–71, 2012.
Butcher, D. T., T. Alliston, and V. M. Weaver. A tense situation: forcing tumour progression. Nat. Rev. Cancer 9:108–122, 2009.
Celli, J. P., I. Rizvi, C. L. Evans, A. O. Abu-Yousif, and T. Hasan. Quantitative imaging reveals heterogeneous growth dynamics and treatment-dependent residual tumor distributions in a three-dimensional ovarian cancer model. J. Biomed. Opt. 15:51603, 2010.
Cress, R. D., Y. S. Chen, C. R. Morris, M. Petersen, and G. S. Leiserowitz. Characteristics of long-term survivors of epithelial ovarian cancer. Obstet. Gynecol. 126:491–497, 2015.
Däster, S., N. Amatruda, D. Calabrese, R. Ivanek, E. Turrini, R. A. Droeser, P. Zajac, C. Fimognari, G. C. Spagnoli, G. Iezzi, V. Mele, and M. G. Muraro. Induction of hypoxia and necrosis in multicellular tumor spheroids is associated with resistance to chemotherapy treatment. Oncotarget 8:1725–1736, 2016.
de Sousa, G. F., S. R. Wlodarczyk, and G. Monteiro. Carboplatin: molecular mechanisms of action associated with chemoresistance. Braz. J. Pharm. Sci. 50:693–701, 2014.
Dong, C., X. Hu, and C. Z. Dinu. Current status and perspectives in atomic force microscopy-based identification of cellular transformation. Int. J. Nanomed. 11:2107–2118, 2016.
Engler, A. J., S. Sen, H. L. Sweeney, and D. E. Discher. Matrix elasticity directs stem cell lineage specification. Cell 126:677–689, 2006.
Gajjar, K., G. Ogden, M. I. Mujahid, and K. Razvi. Symptoms and risk factors of ovarian cancer: a survey in primary care. ISRN Obstet. Gynecol. 2012. https://doi.org/10.5402/2012/754197.
Gossett, D. R., H. T. K. Tse, S. A. Lee, Y. Ying, A. G. Lindgren, O. O. Yang, J. Rao, A. T. Clark, and D. Di Carlo. Hydrodynamic stretching of single cells for large population mechanical phenotyping. PNAS 109:7630–7635, 2012.
Griffith, L. G., and M. A. Swartz. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 7:211, 2006.
Guck, J., R. Ananthakrishnan, T. J. Moon, C. C. Cunningham, and J. Kas. Optical deformability of soft biological dielectrics. Phys. Rev. Lett. 84:5451–5454, 2000.
Guo, M., A. F. Pegoraro, A. Mao, E. H. Zhou, P. R. Arany, Y. Han, D. T. Burnette, M. H. Jensen, K. E. Kasza, J. R. Moore, F. C. Mackintosh, J. J. Fredberg, D. J. Mooney, J. Lippincott-Schwartz, and D. A. Weitz. Cell volume change through water efflux impacts cell stiffness and stem cell fate. PNAS 114:E8618–E8627, 2017.
Hyler, A. R., N. C. Baudoin, M. S. Brown, M. A. Stremler, D. Cimini, R. V. Davalos, and E. M. Schmelz. Fluid shear stress impacts ovarian cancer cell viability, subcellular organization, and promotes genomic instability. PLoS ONE 13:e0194170, 2018.
Ingber, D. E. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20:811–827, 2006.
Iwatsuki, M., K. Mimori, T. Yokobori, H. Ishi, T. Beppu, S. Nakamori, H. Baba, and M. Mori. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 101:293–299, 2010.
Kageyama, K., Y. Onoyama, H. Kogawa, E. Goto, and K. Tanabe. The maximum and minimum water content and cell volume of human erythrocytes in vitro. Biophys. Chem. 34:79–82, 1989.
Ketene, A. N., E. M. Schmelz, P. C. Roberts, and M. Agah. The effects of cancer progression on the viscoelasticity of ovarian cell cytoskeleton structures. Nanomedicine. 8:93–102, 2012.
Kim, K. S., C. H. Cho, E. K. Park, M.-H. Jung, K.-S. Yoon, and H.-K. Park. AFM-detected apoptotic changes in morphology and biophysical property caused by Paclitaxel in Ishikawa and HeLa Cells. PLoS ONE 7:e30066, 2012.
Kumar, S., and V. M. Weaver. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 28:113–127, 2009.
Labidi-Galy, S. I., et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun. 8:1093, 2017.
Margueritat, J., A. Virgone-Carlotta, S. Monnier, H. Delanoë-Ayari, H. C. Mertani, A. Berthelot, Q. Martinet, X. Dagany, C. Rivière, J.-P. Rieu, and T. Dehoux. High-frequency mechanical properties of tumors measured by Brillouin light scattering. Phys. Rev. Lett. 122:018101, 2019.
Mason, T. G., and D. A. Weitz. Optical measurements of frequency-dependent linear viscoelastic moduli of complex fluids. Phys. Rev. Lett. 74:1250–1253, 2018.
McGrail, D. J., Q. M. N. Kieu, and M. R. Dawson. The malignancy of metastatic ovarian cancer cells is increased on soft matrices through a mechanosensitive Rho–ROCK pathway. J. Cell Sci. 127:2621–2626, 2014.
McGrail, D. J., K. M. McAndrews, C. P. Brandenburg, N. Ravikumar, Q. M. Kieu, and M. R. Dawson. Osmotic regulation is required for cancer cell survival under solid stress. Biophys. J. 109:1334–1337, 2015.
McKenzie, A. J., S. R. Hicks, K. V. Svec, H. Naughton, Z. L. Edmunds, and A. K. Howe. The mechanical microenvironment regulates ovarian cancer cell morphology, migration, and spheroid disaggregation. Sci. Rep. 8:7228, 2018.
Moeendarbary, E., L. Valon, M. Fritzsche, A. R. Harris, D. A. Moulding, A. J. Thrasher, E. Stride, L. Mahadevan, and G. T. Charras. The cytoplasm of living cells behaves as a poroelastic material. Nat. Mater. 12:253, 2013.
Novak, C., E. Horst, and G. Mehta. Review: mechanotransduction in ovarian cancer: shearing into the unknown. APL Bioeng. 2:31701, 2018.
Polacheck, W. J., A. E. German, A. Mammoto, D. E. Ingber, and R. D. Kamm. Mechanotransduction of fluid stresses governs 3D cell migration. Proc. Natl. Acad. Sci. U.S.A. 111:2447–2452, 2014.
Polacheck, W. J., M. L. Kutys, J. Yang, J. Eyckmans, Y. Wu, H. Vasavada, K. K. Hirschi, and C. S. Chen. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature 552:258, 2017.
Reid, B. M., J. B. Permuth, and T. A. Sellers. Epidemiology of ovarian cancer: a review. Cancer Biol. Med. 14:9–32, 2017.
Rizvi, I., U. A. Gurkan, S. Tasoglu, N. Alagic, J. P. Celli, L. B. Mensah, Z. Mai, U. Demirci, and T. Hasan. Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. PNAS 110:1974–1983, 2013.
Scarcelli, G., and S. H. Yun. Reply to ‘Water content, not stifness, dominates Brillouin spectroscopy measurements in hydrated materials’. Nat. Methods 15:561–565, 2018.
Scarcelli, G., P. Kim, and S. H. Yun. In vivo measurement of age-related stiffening in the crystalline lens by Brillouin optical microscopy. Biophys. J. 101:1539–1545, 2011.
Scarcelli, G., S. Kling, E. Quijano, R. Pineda, S. Marcos, and S. H. Yun. Brillouin microscopy of collagen crosslinking: noncontact depth-dependent analysis of corneal elastic modulus. Invest. Ophthalmol. Vis. Sci. 54:1418–1425, 2013.
Scarcelli, G., W. J. Polacheck, H. T. Nia, K. Patel, A. J. Grodzinsky, R. D. Kamm, and S. H. Yun. Noncontact three-dimensional mapping of intracellular hydro-mechanical properties by Brillouin microscopy. Nat. Methods 12:1132–1134, 2015.
Schindelin, J., I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, S. Preibisch, C. Rueden, S. Saalfeld, B. Schmid, J.-Y. Tinevez, D. J. White, V. Hartenstein, K. Eliceiri, P. Tomancak, and A. Cardona. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9:676, 2012.
Shao, P., T. G. Seiler, A. M. Eltony, A. Ramier, S. J. J. Kwok, G. Scarcelli, R. P. Ii, and S.-H. Yun. Effects of corneal hydration on Brillouin microscopy in vivo. Invest. Ophthalmol. Vis. Sci. 59:3020–3027, 2018.
Siegel, R. L., K. D. Miller, and A. Jemal. Cancer statistics, 2017. CA. Cancer J. Clin. 67:7–30, 2017.
Swaminathan, V., K. Mythreye, E. T. O’Brien, A. Berchuck, G. C. Blobe, and R. Superfine. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 71:5075–5080, 2011.
Swartz, M. A., N. Iida, E. W. Roberts, S. Sangaletti, M. H. Wong, F. E. Yull, L. M. Coussens, and Y. A. DeClerck. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 72:2473–2480, 2012.
Tan, D. S., R. Agarwal, and S. B. Kaye. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 7:925–934, 2006.
Webb, J. N., J. P. Su, and G. Scarcelli. Mechanical outcome of accelerated corneal crosslinking evaluated by Brillouin microscopy. J. Cataract Refract. Surg. 43:1458–1463, 2017.
Weiswald, L. B., D. Bellet, and V. Dangles-Marie. Spherical cancer models in tumor biology. Neoplasia 17:1–15, 2015.
Wirtz, D., K. Konstantopoulos, and P. C. Searson. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 11:512, 2011.
Wu, P.-H., et al. A comparison of methods to assess cell mechanical properties. Nat. Methods 15:491, 2018.
Wu, P.-J., I. V. Kabakova, J. W. Ruberti, J. M. Sherwood, I. E. Dunlop, C. Paterson, P. Török, and D. R. Overby. Water content, not stiffness, dominates Brillouin spectroscopy measurements in hydrated materials. Nat. Methods 15:561, 2018.
Xu, W., R. Mezencev, B. Kim, L. Wang, J. McDonald, and T. Sulchek. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 7:e46609, 2012.
Zaman, M. H., L. M. Trapani, A. L. Sieminski, D. MacKellar, H. Gong, R. D. Kamm, A. Wells, D. A. Lauffenburger, and P. Matsudaira. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. U.S.A. 103:10889–10894, 2006.
Zhang, J., X. A. Nou, H. Kim, and G. Scarcelli. Brillouin flow cytometry for label-free mechanical phenotyping of the nucleus. Lab Chip 17:663–670, 2017.
Zhou, E. H., X. Trepat, C. Y. Park, G. Lenormand, M. N. Oliver, S. M. Mijailovich, C. Hardin, D. A. Weitz, J. P. Butler, and J. J. Fredberg. Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition. PNAS. 106:10632–10637, 2009.
Funding
This work is supported in part by the National Institutes of Health (R00 CA175292, R33CA204582 and U01CA202177), National Science Foundation (CMMI-1537027). The authors also acknowledge funding from the Burroughs Wellcome Career Award at the Scientific Interface (to KMS).
Conflict of interest
The authors, Christina Conrad, Kelsey M. Gray, Kimberly M. Stroka, Imran Rizvi, and Giuliano Scarcelli declare that they have no conflict of interest.
Ethical Approval
No animal or human studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor James L. McGrath oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Conrad, C., Gray, K.M., Stroka, K.M. et al. Mechanical Characterization of 3D Ovarian Cancer Nodules Using Brillouin Confocal Microscopy. Cel. Mol. Bioeng. 12, 215–226 (2019). https://doi.org/10.1007/s12195-019-00570-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-019-00570-7