Abstract
Introduction
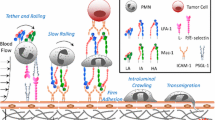
The leukocyte adhesion cascade is important for the maintenance of homeostasis and the ability of immune cells to access sites of infection and inflammation. Despite much work identifying the molecular components of the cascade, and numerous simulations to predict the relationship between molecule density, identity, and adhesion, these relationships have not been measured experimentally.
Methods
Using surfaces functionalized with recombinant ICAM-1 and/or E-selectin along with immobilized SDF-1α, we used a flow chamber to measure rates of tethering, rolling and arrest of primary naïve human CD4+ T lymphocytes on different surface densities of ligand.
Results
Cells required a minimum level of ligand density to progress beyond tethering. E-selectin and ICAM-1 were found to have a synergistic relationship in promoting cell arrest. Surfaces with both ligands had the highest levels of arrest, while surfaces containing only E-selectin hindered the cell’s ability to progress beyond rolling. In contrast, surfaces of ICAM-1 allowed only tethering or arrest. Cells maintained constant rolling velocity and time to stop over large variations in surface density and composition. In addition, surface densities of only O(101) sites/µm2 allowed for rolling while surface densities of O(102) sites/µm2 promoted arrest, approximately equal to previously determined simulated values.
Conclusions
We have systematically and experimentally mapped out the state diagram of T cell adhesion under flow, directly demonstrating the quantitative requirements for each dynamic state of adhesion, and showing how multiple adhesion molecules can act in synergy to secure arrest.



Similar content being viewed by others
References
Alon, R., D. A. Hammer, and T. A. Springer. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature 374:539–542, 1995.
Beste, M. T., and D. A. Hammer. Selectin catch-slip kinetics encode shear threshold adhesive behavior of rolling leukocytes. Proc. Natl. Acad. Sci. U. S. A. 105:20716–20721, 2008.
Bhatia, S. K., M. R. King, and D. A. Hammer. The state diagram for cell adhesion mediated by two receptors. Biophys. J. Elsevier 84:2671–2690, 2003.
Bolomini-Vittori, M., et al. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat. Immunol. 10:185–194, 2009.
Campbell, J. J., J. Hedrick, A. Zlotnik, M. A. Siani, D. A. Thompson, and E. C. Butcher. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 279:381–384, 1998.
Caputo, K. E., D. Lee, M. R. King, and D. A. Hammer. Adhesive dynamics simulations of the shear threshold effect for leukocytes. Biophys. J. 92:787–797, 2007.
Chang, K.-C., and D. A. Hammer. Adhesive dynamics simulations of sialyl-Lewis(x)/E-selectin-mediated rolling in a cell-free system. Biophys. J. 79:1891–1902, 2000.
Chang, K.-C., D. F. J. Tees, and D. A. Hammer. The state diagram for cell adhesion under flow: leukocyte rolling and firm adhesion. Proc. Natl. Acad. Sci. 97:11262–11267, 2000.
Eniola, A. O., P. J. Willcox, and D. A. Hammer. Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs. Biophys. J. 85:2720–2731, 2003.
Evans, E., A. Leung, D. A. Hammer, and S. I. Simon. Chemically distinct transition states govern rapid dissociation of single L-selectin bonds under force. Proc. Natl. Acad. Sci. U. S. A. 98:3784–3789, 2001.
Evans, E., A. Leung, V. Heinrich, and C. Zhu. Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond. Proc. Natl. Acad. Sci. U. S. A. 101:11281–11286, 2004.
Girard, J.-P., C. Moussion, and R. Förster. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 12:762–773, 2012.
Goldman, A. J., R. G. Cox, and H. Brenner. Slow viscous motion of a sphere parallel to a plane wall—II Couette flow. Chem. Eng. Sci. 22:653–660, 1967.
Herter, J., and A. Zarbock. Integrin regulation during leukocyte recruitment. J. Immunol. 190:4451–4457, 2013.
Hidalgo, A., A. J. Peired, M. K. Wild, D. Vestweber, and P. S. Frenette. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity 26:477–489, 2007.
Huang, R. B., and O. Eniola-Adefeso. Shear stress modulation of IL-1β-induced E-selectin expression in human endothelial cells. PLoS ONE 7:1–9, 2012.
Kim, M., C. V. Carman, W. Yang, A. Salas, and T. A. Springer. The primacy of affinity over clustering in regulation of adhesiveness of the integrin αLβ2. J. Cell Biol. 167:1241–1253, 2004.
Kuwano, Y., O. Spelten, H. Zhang, K. Ley, and A. Zarbock. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood 116:617–624, 2010.
Lawrence, M. B., and T. A. Springer. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65:859–873, 1991.
Ley, K., C. Laudanna, M. I. Cybulsky, and S. Nourshargh. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7:678–689, 2007.
McCormick, C. J., A. Craig, D. Roberts, C. I. Newbold, and A. R. Berendt. Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J. Clin. Invest. 100:2521–2529, 1997.
McEver, R. P. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 107:331–339, 2015.
Nourshargh, S., and R. Alon. Leukocyte migration into inflamed tissues. Immunity 41:694–707, 2014.
Salas, A., M. Shimaoka, A. N. Kogan, C. Harwood, U. H. Von Andrian, and T. A. Springer. Rolling adhesion through an extended conformation of integrin alphaLbeta2 and relation to alpha I and beta I-like domain interaction. Immunity 20:393–406, 2004.
San Lek, H., et al. The spontaneously adhesive leukocyte function-associated antigen-1 (LFA-1) integrin in effector T cells mediates rapid actin- and calmodulin-dependent adhesion strengthening to ligand under shear flow. J. Biol. Chem. 288:14698–14708, 2013.
Shamri, R., et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat. Immunol. 6:497–506, 2005.
Shao, B., et al. Signal-dependent slow leukocyte rolling does not require cytoskeletal anchorage of P-selectin glycoprotein ligand-1 (PSGL-1) or integrin αLβ2. J. Biol. Chem. 287:19585–19598, 2012.
Shao, W., et al. AFM investigation on Ox-LDL-induced changes in cell spreading and cell-surface adhesion property of endothelial cells. Scanning 35:119–126, 2013.
Shulman, Z., et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity 30:384–396, 2009.
Simon, S. I., and H. L. Goldsmith. Leukocyte adhesion dynamics in shear flow. Ann. Biomed. Eng. 30:315–332, 2002.
Sundd, P., M. K. Pospieszalska, L. S. L. Cheung, K. Konstantopoulos, and K. Ley. Biomechanics of leukocyte rolling. Biorheology 48:1–35, 2011.
Wright, R. D., and D. Cooper. Glycobiology of leukocyte trafficking in inflammation. Glycobiology 24:1242–1251, 2014.
Yago, T., et al. Blocking neutrophil integrin activation prevents ischemia–reperfusion injury. J. Exp. Med. 212:1267–1281, 2015.
Young, M. E., P. A. Carroad, and R. L. Bell. Estimation of diffusion coefficients of proteins. Biothechnol. Bioeng. 22(5):947–955, 1980.
Acknowledgments
This work was supported by National Institutes of Health Grants to DAH AI082292 and GM123019.
Conflicts of interest
Nicholas R. Anderson, Dooyoung Lee, and Daniel A. Hammer declare that they have no conflicts of interest.
Ethical Standards
No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12195_2018_519_MOESM1_ESM.eps
Supplementary material 1 (EPS 446 kb). Supplementary Fig. 1: Determination of site densities using an IgG1 probe. Protein A/G and SDF-1α were co-immobilized in polystyrene microwells and incubated with IgG1. Site density was determined by comparing to fresh dilutions of an AlexaFluor 488-tagged anti-human IgG1 hinge antibody. Results shown are mean ± SE of three independent experiments
12195_2018_519_MOESM2_ESM.eps
Supplementary material 2 (EPS 4524 kb). Supplementary Fig. 2: Extended state diagrams of the fraction of cells undergoing (A) tethering, (B) rolling, or (C) firm arrest, including surfaces with high site densities. Red dots indicate experimentally tested points. All experiments were performed at a calculated wall shear rate of 100 s−1
12195_2018_519_MOESM3_ESM.eps
Supplementary material 3 (EPS 2541 kb). Supplementary Fig. 3: Comparison between cell spreading after arrest on (A) E-selectin only surfaces and (B) surfaces with E-selectin and ICAM-1. Both images are the end of 10 min flow chamber experiments and are representative of repeated experiments. Orange arrowheads point to arrested cells that are not spread and green arrowheads identify arrested cells that have spread. Surfaces containing only E-selectin show fewer arrested cells than surfaces containing both E-selectin and ICAM-1. Cells arrested on E-selectin surfaces also did not spread efficiently, shown by the fewer cells marked with green. Cells not marked by arrowheads are rolling or tethering during the moment this frame was taken
12195_2018_519_MOESM4_ESM.eps
Supplementary material 4 (EPS 1792 kb). Supplementary Fig. 4: Calculated surface showing the effect of ICAM-1 and E-selectin densities on the distance to stop. Red dots indicate experimentally tested points. All experiments were performed at a calculated wall shear rate of 100 s−1
12195_2018_519_MOESM5_ESM.eps
Supplementary material 5 (EPS 999 kb). Supplementary Fig. 5: Comparison of adhesion on surfaces containing and lacking SDF-1α. Surfaces were functionalized with a 1:1 molar mixture of E-selectin/Fc:ICAM-1/Fc chimeras at an overall site density of 1250 sites/µm2 with and without SDF-1α. Surfaces were compared for their ability to support tethering, rolling, and arrest. Surfaces with SDF-1α showed a rate of arrest approximately 2.5 times higher than surfaces lacking SDF-1α, highlighting the importance of chemokine to cause arrest. Results are the average from three different experiments consisting of duplicate surfaces.
Rights and permissions
About this article
Cite this article
Anderson, N.R., Lee, D. & Hammer, D.A. An Experimentally Determined State Diagram for Human CD4+ T Lymphocyte CXCR4-Stimulated Adhesion Under Shear Flow. Cel. Mol. Bioeng. 11, 91–98 (2018). https://doi.org/10.1007/s12195-018-0519-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-018-0519-x