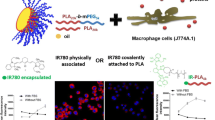
Abstract
Introduction
Intracellular delivery is a key step for many applications in medicine and for investigations into cellular function. This is particularly true for immunotherapy, which often requires controlled delivery of antigen and adjuvants to the cytoplasm of immune cells. Due to the complex responses generated by the stimulation of diverse immune cell populations, it is critical to monitor which cells are targeted during treatment. To address this issue, we have engineered an immunotheranostic polymersome delivery system that fluorescently marks immune cells following intracellular delivery.
Methods
Amine functionalized poly(ethylene glycol)-bl-poly(propylene sulfide) (PEG-PPS-NH2) was synthesized by anionic ring opening polymerization and bridged via perylene bisimide (PBI) to form a tetrablock copolymer (PEG-PPS-PBI-PPS-PEG). Block copolymers were assembled into polymersomes by thin film hydration in phosphate buffered saline and characterized by dynamic light scattering, cryogenic electron microscopy and fluorescence spectroscopy. Polymersomes were injected subcutaneously into the backs of mice, and draining lymph nodes were extracted for flow cytometric analysis of cellular uptake and disassembly.
Results
Modular self-assembly of tetrablock/diblock copolymers in aqueous solutions induced π–π stacking of the PBI linker that both red-shifted and quenched the PBI fluorescence. Reactive oxygen species within the endosomes of phagocytic immune cell populations oxidized the PPS blocks, which disassembled the polymersomes for dequenching and shifting of the PBI fluorescence from 640 to 550 nm emission. Lymph node resident macrophages and dendritic cells were found to increase in 550 nm emission over the course of 3 days by flow cytometry.
Conclusions
Immunotheranostic polymersomes present a versatile platform to probe the contributions of specific cell populations during the elicitation of controlled immune responses. Flanking PBI with two oxidation-sensitive hydrophobic PPS blocks enhanced π stacking and introduced a mechanism for disrupting π–π interactions to shift PBI fluorescence in response to oxidative conditions. Shifts from red (640 nm) to green (550 nm) fluorescence occurred in the presence of physiologically relevant concentrations of reactive oxygen species and could be observed within phagocytic cells both in vitro and in vivo.







Similar content being viewed by others
Abbreviations
- APCs:
-
Antigen presenting cells
- CryoTEM:
-
Cryogenic transmission electron microscopy
- DCs:
-
Dendritic cells
- DLS:
-
Dynamic light scattering
- FBS:
-
Fetal bovine serum
- GPC:
-
Gel permeation chromatography
- MHCI:
-
Major histocompatibility complex I
- MW:
-
Molecular weight
- NK:
-
Natural killer
- PBI:
-
Perylene bisimides
- PTCDA:
-
Perylene-3,4,9,10-tetracarboxylic dianhydride
- PBS:
-
Phosphate-buffered saline
- PDI:
-
Polydispersity index
- PEG-bl-PPS:
-
Poly(ethylene glycol)-bl-poly(propylene sulfide)
- PITC:
-
Polymer-bound isothiocyanate
- ROS:
-
Reactive oxygen species
- SC:
-
Subcutaneous
- TLRs:
-
Toll like receptors
References
Belizaire, R., and E. R. Unanue. Targeting proteins to distinct subcellular compartments reveals unique requirements for MHC class I and II presentation. Proc Natl. Acad. Sci. U.S.A. 106(41):17463–17468, 2009. doi:10.1073/pnas.0908583106.
Berton, G., P. Bellavite, G. de Nicola, P. Dri, and F. Rossi. Plasma membrane and phagosome localisation of the activated NADPH oxidase in elicited peritoneal macrophages of the guinea-pig. J. Pathol. 136(3):241–252, 1982. doi:10.1002/path.1711360307.
Blander, J. M., and R. Medzhitov. On regulation of phagosome maturation and antigen presentation. Nat. Immunol. 7(10):1029–1035, 2006. doi:10.1038/ni1006-1029.
Cerritelli, S., C. P. O’Neil, D. Velluto, A. Fontana, M. Adrian, J. Dubochet, and J. A. Hubbell. Aggregation behavior of poly(ethylene glycol-bl-propylene sulfide) di- and triblock copolymers in aqueous solution. Langmuir 25(19):11328–11335, 2009. doi:10.1021/la900649m.
Chen, Z., V. Stepanenko, V. Dehm, P. Prins, L. D. Siebbeles, J. Seibt, P. Marquetand, V. Engel, and F. Wurthner. Photoluminescence and conductivity of self-assembled pi–pi stacks of perylene bisimide dyes. Chemistry 13(2):436–449, 2007. doi:10.1002/chem.200600889.
Cohn, L., and L. Delamarre. Dendritic cell-targeted vaccines. Front Immunol. 5:255, 2014. doi:10.3389/fimmu.2014.00255.
Delamarre, L., M. Pack, H. Chang, I. Mellman, and E. S. Trombetta. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307(5715):1630–1634, 2005. doi:10.1126/science.1108003.
Dexter, D. L., H. M. Kowalski, B. A. Blazar, Z. Fligiel, R. Vogel, and G. H. Heppner. Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Res. 38(10):3174–3181, 1978.
Dowling, D. J., E. A. Scott, A. Scheid, I. Bergelson, S. Joshi, C. Pietrasanta, S. Brightman, G. Sanchez-Schmitz, S. D. Van Haren, J. Ninkovic, D. Kats, C. Guiducci, A. de Titta, D. K. Bonner, S. Hirosue, M. A. Swartz, J. A. Hubbell, O. Levy. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J. Allergy Clin. Immunol. 2017. doi:10.1016/j.jaci.2016.12.985.
Du, F. F., J. Tian, H. Wang, B. Liu, B. K. Jin, and R. K. Bai. Synthesis and luminescence of POSS-containing perylene bisimide-bridged amphiphilic polymers. Macromolecules 45(7):3086–3093, 2012. doi:10.1021/ma300100s.
Elliott, M. R., K. M. Koster, and P. S. Murphy. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J. Immunol. 198(4):1387–1394, 2017. doi:10.4049/jimmunol.1601520.
Elliott, M. R., and K. S. Ravichandran. Clearance of apoptotic cells: implications in health and disease. J. Cell Biol. 189(7):1059–1070, 2010. doi:10.1083/jcb.201004096.
Gray, E. E., and J. G. Cyster. Lymph node macrophages. J Innate Immun. 4(5–6):424–436, 2012. doi:10.1159/000337007.
Gupta, M. K., T. A. Meyer, C. E. Nelson, and C. L. Duvall. Poly(PS-b-DMA) micelles for reactive oxygen species triggered drug release. J. Control. Release 162(3):591–598, 2012. doi:10.1016/j.jconrel.2012.07.042.
Gvishi, R., and R. Reisfeld. New stable tunable solid-state dye-laser in the red. Optoelectron. Appl. Ind. Med. 1972:390–399, 1993. doi:10.1117/12.151121.
Gvishi, R., R. Reisfeld, and Z. Burshtein. Spectroscopy and laser action of the red perylimide dye in various solvents. Chem. Phys. Lett. 213(3–4):338–344, 1993. doi:10.1016/0009-2614(93)85142-B.
Joffre, O. P., E. Segura, A. Savina, and S. Amigorena. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 12(8):557–569, 2012.
Jouault, N., Y. J. Xiang, E. Moulin, G. Fuks, N. Giuseppone, and E. Buhler. Hierarchical supramolecular structuring and dynamical properties of water soluble polyethylene glycol-perylene self-assemblies. PCCP 14(16):5718–5728, 2012. doi:10.1039/c2cp23786e.
Kaiser, T. E., H. Wang, V. Stepanenko, and F. Wurthner. Supramolecular construction of fluorescent J-aggregates based on hydrogen-bonded perylene dyes. Angew. Chem. Int. Ed. Engl. 46(29):5541–5544, 2007. doi:10.1002/anie.200701139.
Kelkar, S. S., and T. M. Reineke. Theranostics: combining imaging and therapy. Bioconj Chem. 22(10):1879–1903, 2011. doi:10.1021/bc200151q.
Klebe, G., F. Graser, E. Hadicke, and J. Berndt. Crystallochromy as a solid-state effect—correlation of molecular-conformation, crystal packing and color in perylene-3,4-9,10-bis(dicarboximide) pigments. Acta Crystallogr. B 45:69–77, 1989. doi:10.1107/S0108768188010407.
Langhals, H., S. Demmig, and T. Potrawa. The relation between packing effects and solid-state fluorescence of dyes. J. Prakt. Chem. 333(5):733–748, 1991. doi:10.1002/prac.19913330508.
Langhals, H., W. Jona, F. Einsiedl, and S. Wohnlich. Self-dispersion: spontaneous formation of colloidal dyes in water. Adv. Mater. 10(13):1022–1024, 1998. doi:10.1002/(Sici)1521-4095(199809)10:13<1022::Aid-Adma1022>3.3.Co;2-F.
Lennon-Dumenil, A. M., A. H. Bakker, R. Maehr, E. Fiebiger, H. S. Overkleeft, M. Rosemblatt, H. L. Ploegh, and C. Lagaudriere-Gesbert. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J. Exp. Med. 196(4):529–540, 2002.
Li, T. S., and E. Marban. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells 28(7):1178–1185, 2010. doi:10.1002/stem.438.
Lim, E. K., T. Kim, S. Paik, S. Haam, Y. M. Huh, and K. Lee. Nanomaterials for theranostics: recent advances and future challenges. Chem. Rev. 115(1):327–394, 2015.
Lukacs, G. L., O. D. Rotstein, and S. Grinstein. Phagosomal acidification is mediated by a vacuolar-type H(+)-ATPase in murine macrophages. J. Biol. Chem. 265(34):21099–21107, 1990.
Lukacs, G. L., O. D. Rotstein, and S. Grinstein. Determinants of the phagosomal pH in macrophages. In situ assessment of vacuolar H(+)-ATPase activity, counterion conductance, and H+ “leak”. J. Biol. Chem. 266(36):24540–24548, 1991.
Mais, S., J. Tittel, T. Basche, C. Brauchle, W. Gohde, H. Fuchs, G. Muller, and K. Mullen. Terrylenediimide: a novel fluorophore for single-molecule spectroscopy and microscopy from 1.4 K to room temperature. J. Phys. Chem. A 101(45):8435–8440, 1997. doi:10.1021/jp9719063.
Napoli, A., M. J. Boerakker, N. Tirelli, R. J. M. Nolte, N. A. J. M. Sommerdijk, and J. A. Hubbell. Glucose-oxidase based self-destructing polymeric vesicles. Langmuir 20(9):3487–3491, 2004. doi:10.1021/La0357054.
Napoli, A., M. Valentini, N. Tirelli, M. Muller, and J. A. Hubbell. Oxidation-responsive polymeric vesicles. Nat. Mater. 3(3):183–189, 2004. doi:10.1038/nmat1081.
Parker, J. S., and C. M. Perou. Tumor heterogeneity: focus on the leaves, the trees, or the forest? Cancer Cell 28(2):149–150, 2015. doi:10.1016/j.ccell.2015.07.011.
Postow, M. A., J. Chesney, A. C. Pavlick, C. Robert, K. Grossmann, D. McDermott, G. P. Linette, N. Meyer, J. K. Giguere, S. S. Agarwala, M. Shaheen, M. S. Ernstoff, D. Minor, A. K. Salama, M. Taylor, P. A. Ott, L. M. Rollin, C. Horak, P. Gagnier, J. D. Wolchok, and F. S. Hodi. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 372(21):2006–2017, 2015. doi:10.1056/NEJMoa1414428.
Savina, A., and S. Amigorena. Phagocytosis and antigen presentation in dendritic cells. Immunol. Rev. 219:143–156, 2007. doi:10.1111/j.1600-065X.2007.00552.x.
Savina, A., C. Jancic, S. Hugues, P. Guermonprez, P. Vargas, I. C. Moura, A. M. Lennon-Dumenil, M. C. Seabra, G. Raposo, and S. Amigorena. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126(1):205–218, 2006. doi:10.1016/j.cell.2006.05.035.
Scott, E. A., A. Stano, M. Gillard, A. C. Maio-Liu, M. A. Swartz, and J. A. Hubbell. Dendritic cell activation and T cell priming with adjuvant- and antigen-loaded oxidation-sensitive polymersomes. Biomaterials 33(26):6211–6219, 2012. doi:10.1016/j.biomaterials.2012.04.060.
Stano, A., E. A. Scott, K. Y. Dane, M. A. Swartz, and J. A. Hubbell. Tunable T cell immunity towards a protein antigen using polymersomes vs. solid-core nanoparticles. Biomaterials 34(17):4339–4346, 2013. doi:10.1016/j.biomaterials.2013.02.024.
Vasdekis, A. E., E. A. Scott, C. P. O’Neil, D. Psaltis, and J. A. Hubbell. Precision intracellular delivery based on optofluidic polymersome rupture. ACS Nano 6(9):7850–7857, 2012. doi:10.1021/nn302122h.
Voigt, J., K. Hunniger, M. Bouzani, I. D. Jacobsen, D. Barz, B. Hube, J. Loffler, and O. Kurzai. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J. Infect. Dis. 209(4):616–626, 2014. doi:10.1093/infdis/jit574.
Vulcano, M., S. Dusi, D. Lissandrini, R. Badolato, P. Mazzi, E. Riboldi, E. Borroni, A. Calleri, M. Donini, A. Plebani, L. Notarangelo, T. Musso, and S. Sozzani. Toll receptor-mediated regulation of NADPH oxidase in human dendritic cells. J. Immunol. 173(9):5749–5756, 2004.
Wagner, C. S., J. Grotzke, and P. Cresswell. Intracellular regulation of cross-presentation during dendritic cell maturation. PLoS ONE 8(10):e76801, 2013. doi:10.1371/journal.pone.0076801.
Wurthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 14:1564–1579, 2004. doi:10.1039/b401630k.
Würthner, F., C. Bauer, V. Stepanenko, and S. Yagai. A black perylene bisimide super gelator with an unexpected J-type absorption band. Adv. Mater. 20(9):1695–1698, 2008. doi:10.1002/adma.200702935.
Yamaguchi, T., and M. Kaneda. Presence of cytochrome b-558 in guinea-pig alveolar macrophages-subcellular localization and relationship with NADPH oxidase. Biochim. Biophys. Acta 933(3):450–459, 1988.
Yi, S., S. D. Allen, Y. G. Liu, B. Z. Ouyang, X. Li, P. Augsornworawat, E. B. Thorp, and E. A. Scott. Tailoring nanostructure morphology for enhanced targeting of dendritic cells in atherosclerosis. ACS Nano 10(12):11290–11303, 2016. doi:10.1021/acsnano.6b06451.
Zhang, X., Z. Chen, and F. Wurthner. Morphology control of fluorescent nanoaggregates by co-self-assembly of wedge- and dumbbell-shaped amphiphilic perylene bisimides. J. Am. Chem. Soc. 129(16):4886–4887, 2007. doi:10.1021/ja070994u.
Acknowledgments
We would like to thank J. Remis for CryoTEM assistance and the following facilities at Northwestern University: Robert H. Lurie Comprehensive Cancer Center Flow Cytometry Core; Center for Advanced Molecular Imaging; Biological imaging facility; Mouse Histology and Phenotyping Laboratory; and the Keck Interdisciplinary Surface Science Facility. This work was supported by the National Institutes of Health Director’s New Innovator Award (grant no. 1DP2HL132390-01), the Louis A. Simpson & Kimberly K. Querrey Center for Regenerative Nanomedicine Regenerative Nanomedicine Catalyst Award.
Conflict of Interest
Fanfan Du, Yu-Gang Liu, and Evan A. Scott declare that they have no conflicts of interest.
Ethical Standards
No human studies were carried out by the authors for this article. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the Northwestern University Institutional Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Evan Alexander Scott completed his undergraduate degree in Biomedical Engineering at Brown University in 2002. After working for a year as a chemical and biological defense engineer at the Battelle Memorial Institute in Aberdeen MD, he obtained a Ph.D. in Biomedical Engineering in 2009 from Washington University in St. Louis. His dissertation work was performed in the laboratory of Prof. Donald Elbert, where he developed methods based in proteomics and polymer chemistry to both analyze and control the interactions between blood and the material surfaces of cardiovascular devices. As a Whitaker International Scholar, he performed postdoctoral research in Switzerland in the laboratories of Prof. Jeffrey Hubbell and Prof. Melody Swartz at the École Polytechnique Fédérale de Lausanne (EPFL) from 2009 to 2013. There he focused on the development of nanomaterial-based formulations and strategies for both neonatal vaccination and cancer immunotherapy. Dr. Scott joined Northwestern University as a tenure-track Assistant Professor of Biomedical Engineering in the fall of 2013. His immunoengineering laboratory applies principles from biomaterials science, nanotechnology and tissue engineering towards the development of translational immunotherapies for heart disease and the rational design of vaccines for cancer and infectious diseases.

This paper is part of the 2017 Young Innovators Issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Du, F., Liu, YG. & Scott, E.A. Immunotheranostic Polymersomes Modularly Assembled from Tetrablock and Diblock Copolymers with Oxidation-Responsive Fluorescence. Cel. Mol. Bioeng. 10, 357–370 (2017). https://doi.org/10.1007/s12195-017-0486-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-017-0486-7