Abstract
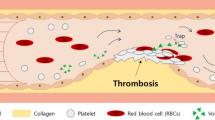
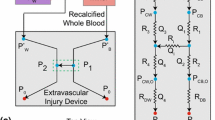
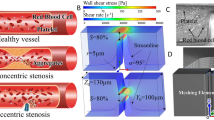
The reaction dynamics of a complex mixture of cells and proteins, such as blood, in branched circulatory networks within the human microvasculature or extravascular therapeutic devices such as extracorporeal oxygenation machine (ECMO) remains ill-defined. In this report we utilize a multi-bypass microfluidics ladder network design with dimensions mimicking venules to study patterns of blood platelet aggregation and fibrin formation under complex shear. Complex blood fluid dynamics within multi-bypass networks under flow were modeled using COMSOL. Red blood cells and platelets were assumed to be non-interacting spherical particles transported by the bulk fluid flow, and convection of the activated coagulation factor II, thrombin, was assumed to be governed by mass transfer. This model served as the basis for predicting formation of local shear rate gradients, stagnation points and recirculation zones as dictated by the bypass geometry. Based on the insights from these models, we were able to predict the patterns of blood clot formation at specific locations in the device. Our experimental data was then used to adjust the model to account for the dynamical presence of thrombus formation in the biorheology of blood flow. The model predictions were then compared to results from experiments using recalcified whole human blood. Microfluidic devices were coated with the extracellular matrix protein, fibrillar collagen, and the initiator of the extrinsic pathway of coagulation, tissue factor. Blood was perfused through the devices at a flow rate of 2 µL/min, translating to physiologically relevant initial shear rates of 300 and 700 s−1 for main channels and bypasses, respectively. Using fluorescent and light microscopy, we observed distinct flow and thrombus formation patterns near channel intersections at bypass points, within recirculation zones and at stagnation points. Findings from this proof-of-principle ladder network model suggest a specific correlation between microvascular geometry and thrombus formation dynamics under shear. This model holds potential for use as an integrative approach to identify regions susceptible to intravascular thrombus formation within the microvasculature as well as extravascular devices such as ECMO.







Similar content being viewed by others
Abbreviations
- PDMS:
-
Polydimethylsiloxane
- RBCs:
-
Red blood cells
- TF:
-
Tissue factor
- VWF:
-
Von Willebrand factor
- DiOC6 :
-
3,3′-Dihexyloxacarbocyanine iodide
References
Aird, W. C. Vascular bed-specific thrombosis. J. Thromb. Haemost. 5:283–291, 2007.
Baker-Groberg, S. M., F. A. Cianchetti, K. G. Phillips, and O. J. T. McCarty. Development of a method to quantify platelet adhesion and aggregation under static conditions. Cell. Mol. Bioeng. 7:285–290, 2014.
Baker-Groberg, S. M., S. Lattimore, M. Recht, O. J. T. McCarty, and K. M. Haley. Assessment of neonatal platelet adhesion, activation, and aggregation. J. Thromb. Haemost. 14:815–827, 2016.
Bark, D. L., and D. N. Ku. Platelet transport rates and binding kinetics at high shear over a thrombus. Biophys. J. 105:502–511, 2013.
Bird, R. B., W. E. Stewart, and E. N. Lightfoot. Transport Phenomena. New York: Wiley, 1960.
Boyer, C. J., and R. D. Swartz. Severe clotting during extracorporeal dialysis procedures. Sem. Dial. 4:69–71, 1991.
Brækkan, S. K., E. B. Mathiesen, I. Njølstad, T. Wilsgaard, and J.-B. Hansen. Hematocrit and risk of venous thromboembolism in a general population. The Tromsø study. Haematologica 95:270–275, 2010.
Burgin, T. D., H. Johnson, A. Chung, and J. McGrath. Analytical and finite element modeling of nanomembranes for miniaturized, continuous hemodialysis. Membranes (Basel) 6(1):6, 2015.
Carson, L., and V. M. Doctor. Mechanism of potentiation of antithrombin III and heparin cofactor II inhibition by sulfated xylans. Thromb. Res. 58:367–381, 1990.
Chang, J. Y. Thrombin specificity. Requirement for apolar amino acids adjacent to the thrombin cleavage site of polypeptide substrate. Eur. J. Biochem. 151:217–224, 1985.
Chiu, W.-C., M. J. Slepian, and D. Bluestein. Thrombus formation patterns in the HeartMate II ventricular assist device: clinical observations can be predicted by numerical simulations. ASAIO J. 60:237–240, 2014.
Colace, T. V., G. W. Tormoen, O. J. T. McCarty, and S. L. Diamond. Microfluidics and coagulation biology. Annu. Rev. Biomed. Eng. 15:283–303, 2013.
Doshi, N., J. N. Orje, B. Molins, J. W. Smith, S. Mitragotri, and Z. M. Ruggeri. Platelet mimetic particles for targeting thrombi in flowing blood. Adv. Mater. Weinh. 24:3864–3869, 2012.
Eckstein, E. C., and F. Belgacem. Model of platelet transport in flowing blood with drift and diffusion terms. Biophys. J. 60:53–69, 1991.
Flamm, M. H., and S. L. Diamond. Multiscale systems biology and physics of thrombosis under flow. Ann. Biomed. Eng. 40:2355–2364, 2012.
Fogelson, A. L., and K. B. Neeves. Fluid mechanics of blood clot formation. Ann. Rev. Fluid Mech. 47:377–403, 2015.
Hammon, J.W. Extracorporeal circulation. In: Cardiac Surgery in the Adult, 4th ed. New York: McGraw-Hill Medical, 2008, pp. 350–370.
Han, X., R. Bibb, and R. Harris. Artificial vascular bifurcations—design and modelling. Proc. CIRP 49:14–18, 2016.
Hansen, R. R., A. R. Wufsus, S. T. Barton, A. A. Onasoga, R. M. Johnson-Paben, and K. B. Neeves. High content evaluation of shear dependent platelet function in a microfluidic flow assay. Ann. Biomed. Eng. 41:250–262, 2013.
Hathcock, J. J. Flow effects on coagulation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 26:1729–1737, 2006.
Holme, P. A., et al. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler. Thromb. Vasc. Biol. 17:646–653, 1997.
Hussain, M. A., S. Kar, and R. R. Puniyani. Relationship between power law coefficients and major blood constituents affecting the whole blood viscosity. J. Biosci. 24:329–337, 1999.
Jackson, S. P., W. S. Nesbitt, and E. Westein. Dynamics of platelet thrombus formation. J. Thromb. Haemost. 7:17–20, 2009.
Jain, A., A. Graveline, A. Waterhouse, A. Vernet, R. Flaumenhaft, and D. E. Ingber. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat. Commun. 7:10176, 2016.
Jain, A., and L. L. Munn. Biomimetic postcapillary expansions for enhancing rare blood cell separation on a microfluidic chip. Lab Chip 11:2941–2947, 2011.
Johnson-Chavarria, E. M., M. Tanyeri, and C. M. Schroeder. A microfluidic-based hydrodynamic trap for single particles. J. Vis. Exp. 47:e2517, 2011.
Johnston, B. M., P. R. Johnston, S. Corney, and D. Kilpatrick. Non-Newtonian blood flow in human right coronary arteries: steady state simulations. J. Biomech. 37:709–720, 2004.
Jønsson, V., J. E. Bock, and J. B. Nielsen. Significance of plasma skimming and plasma volume expansion. J. Appl. Physiol. 72:2047–2051, 1992.
Kang, E., D. H. Lee, C.-B. Kim, S. J. Yoo, and S.-H. Lee. A hemispherical microfluidic channel for the trapping and passive dissipation of microbubbles. J. Micromech. Microeng. 20:45009, 2010.
Khandelwal, V., A. Dhiman, and L. Baranyi. Laminar flow of non-Newtonian shear-thinning fluids in a T-channel. Comput. Fluids 108:79–91, 2015.
Kniazeva, T., J. C. Hsiao, J. L. Charest, and J. T. Borenstein. A microfluidic respiratory assist device with high gas permeance for artificial lung applications. Biomed. Microdevices 13:315–323, 2011.
Lasheras, J. C. The biomechanics of arterial aneurysms. Annu. Rev. Fluid Mech. 39:293–319, 2007.
Lee, S. Y. K., M. Wong, and Y. Zohar. Pressure losses in microchannels with bends. In: The 14th IEEE International Conference on Micro Electro Mechanical Systems, 2001. MEMS 2001, pp. 491–494, 2001.
Lee, A. M., G. W. Tormoen, E. Kanso, O. J. T. McCarty, and P. K. Newton. Modeling and simulation of procoagulant circulating tumor cells in flow. Front. Oncol. 2:108, 2012.
Lei, M., C. Kleinstreuer, and G. A. Truskey. Numerical investigation and prediction of atherogenic sites in branching arteries. J. Biomech. Eng. 117:350–357, 1995.
Liberale, C., et al. Integrated microfluidic device for single-cell trapping and spectroscopy. Sci. Rep. 3:1258, 2013.
Liu, L., et al. Inhibition of thrombin by antithrombin III and heparin cofactor II in vivo. Thromb. Haemost. 73:405–412, 1995.
Mackman, N. New insights into the mechanisms of venous thrombosis. J. Clin. Investig. 122:2331–2336, 2012.
Maddala, J., W. S. Wang, S. A. Vanapalli, and R. Rengaswamy. Traffic of pairs of drops in microfluidic ladder networks with fore-aft structural asymmetry. Microfl. Nanofl. 14:337–344, 2013.
Madershahian, N., R. Nagib, J. Wippermann, J. Strauch, and T. Wahlers. A simple technique of distal limb perfusion during prolonged femoro-femoral cannulation. J. Card. Surg. 21:168–169, 2006.
McDonald, J. C., et al. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 21:27–40, 2000.
Meyer, A., M. Strüber, and S. Fischer. Advances in extracorporeal ventilation. Anesth. Clinics 26:381–391, 2008.
Nesbitt, W. S., et al. A shear gradient–dependent platelet aggregation mechanism drives thrombus formation. Nat. Medicine 15:665–673, 2009.
Nosovitsky, V. A., O. J. Ilegbusi, J. Jiang, P. H. Stone, and C. L. Feldman. Effects of curvature and stenosis-like narrowing on wall shear stress in a coronary artery model with phasic flow. Comp. Biomed. Res. 30:61–82, 1997.
Pearson, T. C., and G. Wetherley-Mein. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet 2:1219–1222, 1978.
Perkkiö, J., L. J. Wurzinger, and H. Schmid-Schönbein. Plasma and platelet skimming at T-junctions. Thromb. Res. 45:517–526, 1987.
Phillips, K. G., et al. Optical quantification of cellular mass, volume, and density of circulating tumor cells identified in an ovarian cancer patient. Front. Oncol. 2:72, 2012.
Popel, A. S., and P. C. Johnson. Microcirculation and hemorheology. Annu. Rev. Fluid Mech. 37:43–69, 2005.
Rana, K., B. J. Timmer, and K. B. Neeves. A combined microfluidic-microstencil method for patterning biomolecules and cells. Biomicrofluidics 8:56502, 2014.
Replogle, R. L., H. J. Meiselman, and E. W. Merrill. Clinical implications of blood rheology studies. Circulation 36:148–160, 1967.
Runyon, M. K., C. J. Kastrup, B. L. Johnson-Kerner, T. G. Van Ha, and R. F. Ismagilov. Effects of shear rate on propagation of blood clotting determined using microfluidics and numerical simulations. J. Am. Chem. Soc. 130:3458–3464, 2008.
Shankaran, H., P. Alexandridis, and S. Neelamegham. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood 101:2637–2645, 2003.
Tousi, N., B. Wang, K. Pant, M. F. Kiani, and B. Prabhakarpandian. Preferential adhesion of leukocytes near bifurcations is endothelium independent. Microvasc. Res. 80:384–388, 2010.
Tsai, M., et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J. Clin. Investig. 122:408–418, 2012.
Turitto, V. T., and H. R. Baumgartner. Platelet interaction with subendothelium in a perfusion system: physical role of red blood cells. Microvasc. Res. 9:335–344, 1975.
Turitto, V. T., and H. J. Weiss. Red blood cells: their dual role in thrombus formation. Science 207:541–543, 1980.
Vollmer, A. P., R. F. Probstein, R. Gilbert, and T. Thorsen. Development of an integrated microfluidic platform for dynamic oxygen sensing and delivery in a flowing medium. Lab Chip 5:1059–1066, 2005.
Walker, C. P. R., and D. Royston. Thrombin generation and its inhibition: a review of the scientific basis and mechanism of action of anticoagulant therapies. Br. J. Anaesth. 88:848–863, 2002.
Watts, T., M. Barigou, and G. B. Nash. Comparative rheology of the adhesion of platelets and leukocytes from flowing blood: why are platelets so small? Am. J. Physiol. Heart Circ. Physiol. 304:H1483–H1494, 2013.
Westein, E., A. D. van der Meer, M. J. E. Kuijpers, J.-P. Frimat, A. van den Berg, and J. W. M. Heemskerk. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc. Natl. Acad. Sci. USA 110:1357–1362, 2013.
White-Adams, T. C., et al. Laminin promotes coagulation and thrombus formation in a factor XII-dependent manner. J. Thromb. Haemost. 8:1295–1301, 2010.
Wu, W.-I., et al. Lung assist device: development of microfluidic oxygenators for preterm infants with respiratory failure. Lab Chip 13:2641–2650, 2013.
You, J., L. Flores, M. Packirisamy, and I. Stiharu. Modeling the effect of channel bends on microfluidic flow. In: IASME Transactions Udine, pp. 144–151, 2005.
Zheng, Y., J. Chen, and J. A. López. Flow-driven assembly of VWF fibres and webs in in vitro microvessels. Nat. Commun. 6:7858, 2015.
Zilberman-Rudenko, J., et al. Biorheology of platelet activation in the bloodstream distal to thrombus formation. Cell. Mol. Bioeng. 9:1–13, 2016.
Zilberman-Rudenko, J., et al. Coagulation factor XI promotes distal platelet activation and single platelet consumption in the bloodstream under shear flow. Arterioscler. Thromb. Vasc. Biol. 36:510–517, 2016.
Zimmerman, W. B. J. Microfluidics: History, Theory and Applications. New York: Springer, 2006.
Acknowledgments
We thank Dr. András Gruber for insightful comments and Chantal Wiesenekker for technical assistance. This work was supported by West Virginia University startup funds awarded to J. Maddala and by grants from the National Institutes of Health (R01HL101972, R01GM116184, R44HL126235). O.J.T. McCarty is an American Heart Association Established Investigator (13EIA12630000).
Conflict of interest
J. Zilberman-Rudenko, J.L. Sylman, H.H.S. Lakshman, O.J.T. McCarty and J. Maddala declare no competing financial interests.
Human and Animal Rights and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study. All human subject research was carried out in accordance with institutional guidelines approved by the Oregon Health & Science University Institutional Review Board. No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
O. J. T. McCarty and J. Maddala are co-senior authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Video 1
Initial blood flow dynamics within the ladder microfluidic device. Recalcified whole human blood was perfused at a 2 µL/min flow rate through a PDMS-ladder network coated with collagen and tissue factor; real-time video of the blood flow was recorded differential using interference contrast, DIC, microscopy. Supplementary material 1 (WMV 4603 kb)
Supplemental Video 2
Blood flow dynamics within the ladder microfluidic device after 10 min of blood perfusion. Recalcified whole human blood was perfused at a 2 µL/min flow rate through a PDMS-ladder network coated with collagen and tissue factor; real-time video of the blood flow was recorded differential using interference contrast, DIC, microscopy at the 10 min time point. Supplementary material 2 (WMV 447 kb)
Supplemental Fig. 1
Quantification of the spatial distribution of thrombus formation. DiOC6-labeled whole human blood was perfused at a 2 µL/min flow rate through a PDMS-ladder network coated with collagen and tissue factor; images of thrombus formation were recorded using differential interference contrast, DIC microscopy. The thrombus surface area was quantified for 5 experiments using ImageJ. Supplementary material 3 (TIFF 976 kb)
Rights and permissions
About this article
Cite this article
Zilberman-Rudenko, J., Sylman, J.L., Lakshmanan, H.H.S. et al. Dynamics of Blood Flow and Thrombus Formation in a Multi-Bypass Microfluidic Ladder Network. Cel. Mol. Bioeng. 10, 16–29 (2017). https://doi.org/10.1007/s12195-016-0470-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-016-0470-7