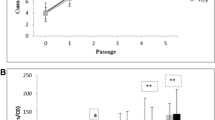
Abstract
The aim of this work was to investigate the effects of various combinations of a static magnetic field and iron oxide nanoparticles on the growth, proliferation rate, morphology and ultrastructure of equine adipose-derived mesenchymal stem cells (ASCs). Cell migration patterns were also evaluated in order to determine whether the stem cells could be attracted using a magnetic field after iron oxide uptake. Proliferation activity of cells after magnetic field and iron oxide treatment was determined with a resazurin based cytotoxic assay whereas morphology, cytophisiological activity and ultrastructure were evaluated utilizing light, fluorescent and scanning electron microscopy. Morphological and ultrastructural examination of equine ASCs showed that exposure to a magnetic field did not cause any significant changes in cell morphology, however a shift of nuclei to the peripheral parts of the cell was observed in ultrastructural examinations. Although the cells had a lower proliferation factor after the uptake of iron nanoparticles, they could still be attracted to the desired area using a static magnetic field. This method may allow ASCs to be directed and retained within the desired area. These findings support the potential value of the combination of static magnetic fields and iron oxide nanoparticles in the treatment of equine injures.







Similar content being viewed by others
References
Ahmadinejad, M., J. Pishkar, H. Pashzanoussi, and S. Tavakoli. Common injuries in athletic horses at riding clubs in Tehran, Iran. Int. J. Vet. Res. 5(1):17–20, 2011.
Bekhite, M., A. Finkensieper, F. A. Abou-Zaid, I. K. El-Shourbagy, K. M. Omar, H. R. Figulla, H. Sauer, and M. Wartenberg. Static electromagnetic fields induce vasculogenesis and chondro-osteogenesis of mouse embryonic stem cells by reactive oxygen species-mediated up-regulation of vascular endothelial growth factor. Stem Cells Dev. 19(5):731–743, 2010.
Biancone, L., S. Bruno, M. C. Deregibus, C. Tetta, and G. Camussi. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol. Dial. Transplant. 27:3037–3042, 2012.
Carvalho, A. D., P. R. Badial, L. E. Alvarez, A. L. Yamada, A. S. Borges, E. Deffune, C. A. Hussni, and A. L. Garcia Alves. Equine tendonitis therapy using mesenchymal stem cells and platelet concentrates: a randomized controlled trial. Stem Cell Res. Ther. 4(4):85, 2013.
Colbert, A. P., H. Wahbeh, N. Harling, E. Connelly, H. C. Schiffke, C. Forsten, W. L. Gregory, M. S. Markov, J. J. Souder, P. Elmer, and V. King. Static magnetic field therapy: a critical review of treatment parameters. Evid. Based Complement. Alternat. Med. 6(2):133–139, 2009.
Esposito, M., A. Lucariello, C. Costanzo, A. Fiumarella, A. Giannini, G. Riccardi, and I. Riccio. Differentiation of human umbilical cord-derived mesenchymal stem cells, WJ-MSCs, into chondrogenic cells in the presence of pulsed electromagnetic fields. In Vivo 27(4):495–500, 2013.
Gimble, J. M., A. J. Katz, and B. A. Bunnell. Adipose-derived stem cells for regenerative medicine. Circ. Res. 100(9):1249–1260, 2007.
Godwin, E. E., N. J. Young, J. Dudhia, I. C. Beamish, and R. K. Smith. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet. J. 44:25–32, 2012.
Grzesiak, J., K. Marycz, J. Czogała, K. Wrzeszcz, and J. Nicpoń. Comparison of behavior, morphology and morphometry of equine and canine adipose derived mesenchymal stem cells in culture. Int. J. Morphol. 29:1012–1017, 2011.
Grzesiak, J., K. Marycz, D. Szarek, and W. Jarmundowicz. Morphological characterization of gecko’s (Eublepharis macularius) glial cells in culture. Int. J. Morphol. 31(3):826–831, 2013.
Guest, D. J., M. R. Smith, and W. R. Allen. Monitoring the fate of autologous and allogeneic mesenchymal progenitor cells injected into the superficial digital flexor tendon of horses: preliminary study. Equine Vet. J. 40:178–181, 2008.
Hattori, H., K. Masuoka, M. Sato, M. Ishihara, T. Asazuma, B. Takase, M. Kikuchi, K. Nemoto, and M. Ishihara. Bone formation using human adipose tissue-derived stromal cells and a biodegradable scaffold. J. Biomed. Mater. Res. B 76(1):230–239, 2006.
Hill, T., D. Carmichael, G. Maylin, and L. Krook. Track condition and racing injuries in thoroughbred horses. Cornell Vet. 76(4):361–379, 1986.
Hsu, S. H., and J. C. Chang. The static magnetic field accelerates the osteogenic differentiation and mineralization of dental pulp cells. Cytotechnology 2:143–155, 2010.
Jasmin, A. L. Torres, H. M. Nunes, J. A. Passipieri, L. A. Jelicks, E. L. Gasparetto, D. C. Spray, A. C. Campos de Carvalho, and R. Mendez-Otero. Optimized labeling of bone marrow mesenchymal cells with superparamagnetic iron oxide nanoparticles and in vivo visualization by magnetic resonance imaging. J. Nanobiotechnol. 9:4, 2011.
Kotani, H., H. Kawaguchi, T. Shimoaka, M. Iwasaka, S. Ueno, H. Ozawa, K. Nakamura, and K. Hoshi. Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J. Bone Miner. Res. 17(10):1814–1821, 2002.
Lacy-Hulbert, A., J. C. Metcalfe, and R. Hesketh. Biological responses to electromagnetic fields. FASEB J. 12(6):395–420, 1998.
Lai, R. C., T. S. Chen, and S. K. Lim. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen. Med. 6:481–492, 2011.
Lee, S. W., P. Padmanabhan, and P. Ray. Stem cell-mediated accelerated bone healing observed with in vivo molecular and small animal imaging technologies in a model of skeletal injury. J Orthop. Res. 27:295–302, 2009.
Luo, F., T. Hou, Z. Zhang, Z. Xie, X. Wu, and J. Xu. Effects of pulsed electromagnetic field frequencies on the osteogenic differentiation of human mesenchymal stem cells. Orthopedics 35(4):526–531, 2012.
Marędziak, M., K. Marycz, A. Śmieszek, D. Lewandowski, and N. Y. Toker. The influence of static magnetic fields on canine and equine mesenchymal stem cells derived from adipose tissue. In Vitro Cell Dev. Biol. Anim. 50(6):562–571, 2014.
Markov, M. S. Therapeutic application of static magnetic fields. Environmentalist 27:457–463, 2007.
Marycz, K., J. Grzesiak, K. Wrzeszcz, and P. Golonka. Adipose stem cell combined with plasma-based implant bone tissue differentiation in vitro and in a horse with a phalanx digitalis distalis fracture: a case report. Vet. Med. 57(11):610–617, 2012.
Marycz, K., A. Śmieszek, J. Grzesiak, A. Donesz-Sikorska, and J. Krzak-Roś. Application of bone marrow and adipose-derived stem cells for testing the biocompatibility of metal-based biomaterials functionalized with ascorbic acid. Biomed. Mater. 8(6):065004, 2013.
Marycz, K., N. Y. Toker, J. Grzesiak, K. Wrzeszcz, and P. Golonka. The therapeutic effect of autogenic adipose derived stem cells combined with autogenic platelet rich plasma in tendons disorders in horses in vitro and in vivo research. J. Anim. Vet. Adv. 11(23):4324–4331, 2012.
Marycz, K., N. Y. Toker, A. Śmieszek, and J. Nicpoń. The morphology and proliferation rate of canine and equine adipose derived stem cells cultured with flunixin meglumine—in vitro. Kafkas Univ. Vet. Fak. Derg. 9:1–7, 2013.
Mizuno, H., M. Tobita, and A. C. Uysal. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 30(5):804–810, 2012.
Nicpoń, J., K. Marycz, and J. Grzesiak. Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Pol. J. Vet. Sci. 16(4):753–754, 2013.
Pesce, M., A. Patruno, L. Speranza, and M. Reale. Extremely low frequency electromagnetic field and wound healing: implication of cytokines as biological mediators. Eur. Cytokine Netw. 24(1):1–10, 2013.
Pislaru, S. V., A. Harbuzariu, and R. Gulati. Magnetically targeted endothelial cell localization in stented vessels. J. Am. Coll. Cardiol. 48:1839–1845, 2006.
Ross, C. L., and B. S. Harrison. The use of magnetic field for the reduction of inflammation: a review of the history and therapeutic results. Altern. Ther. Health Med. 19(2):47–54, 2013.
Sato, K., H. Yamaguchi, H. Miyamoto, and Y. Kinouchi. Growth of human cultured cells exposed to a non-homogeneous static magnetic field generated by Sm-Co magnets. Biochim. Biophys. Acta 1136(3):231–238, 1992.
Schäfer, R., R. Bantleon, R. Kehlbach, G. Siegel, J. Wiskirchen, H. Wolburg, T. Kluba, F. Eibofner, H. Northoff, C. D. Claussen, and H. P. Schlemmer. Functional investigations on human mesenchymal stem cells exposed to magnetic fields and labeled with clinically approved iron nanoparticles. BMC Cell Biol. 11:22, 2010.
Schäfer, R., R. Kehlbach, M. Müller, R. Bantleon, T. Kluba, M. Ayturan, G. Siegel, H. Wolburg, H. Northoff, K. Dietz, C. D. Claussen, and J. Wiskirchen. Labeling of human mesenchymal stromal cells with superparamagnetic iron oxide leads to a decrease in migration capacity and colony formation ability. Cytotherapy 11(1):68–78, 2009.
Tenuzzo, B., A. Chionna, E. Panzarini, R. Lanubile, P. Tarantino, B. Di Jeso, M. Dwikat, and L. Dini. Biological effects of 6 mT static magneticfields: a comparative study in different cell types. Bioelectromagnetics 27(7):560–577, 2006.
Tetta, C., A. L. Consiglio, S. Bruno, E. Tetta, E. Gatti, M. Dobreva, F. Cremonesi, and G. Camussi. The role of microvesicles derived from stem cells in tissue regeneration; a dream for tendon repair? Muscles Ligaments Tendons J. 2(3):212–221, 2012.
Torbet, J., and M. C. Ronzière. Magnetic alignment of collagen during self-assembly. Biochem. J. 219(3):1057–1059, 1984.
Vergallo, C., L. Dini, Z. Szamosvölgyi, B. A. Tenuzzo, E. Carata, E. Panzarini, and J. F. Laszlo. In vitro analysis of the anti-inflammatory effect of inhomogeneous static magnetic field-exposure on human macrophages and lymphocytes. PLoS One 2013. doi:10.1371/journal.pone.0072374.
Yamamoto, Y., Y. Ohsaki, T. Goto, A. Nakasima, and T. Iijima. Effects of static magnetic fields on bone formation in rat osteoblast cultures. J. Dent. Res. 82(12):962–966, 2003.
Acknowlegments
The research was supported by Wroclaw Research Centre EIT+ under the Project “Biotechnologies and Advanced Medical Technologies” – BioMed (POIG.01.01.02-02-003/08) financed from the European Regional Development Fund (Operational Programmed Innovative Economy, 1.1.2.). Publication supported by Wrocław Centre of Biotechnology, programme the Leading National Research Centre (KNOW) for years 2014–2018.
Conflict of interests
Monika Marędziak, Krzysztof Marycz, Agnieszka Śmieszek, and Daniel Lewandowski declare that they have no conflicts of interest.
Ethical Standards
All animal studies were approved by the Second Local Ethic Commission (Chelmonskiego 38C, 51-630 Wroclaw, Poland; decision No. 84/2012). No human subjects research was performed by the authors for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Marędziak, M., Marycz, K., Śmieszek, A. et al. An In Vitro Analysis of Pattern Cell Migration of Equine Adipose Derived Mesenchymal Stem Cells (EqASCs) Using Iron Oxide Nanoparticles (IO) in Static Magnetic Field. Cel. Mol. Bioeng. 8, 566–576 (2015). https://doi.org/10.1007/s12195-015-0402-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-015-0402-y