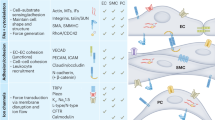
Abstract
Smooth muscle cell (SMC) invasion into plaques and subsequent proliferation is a major factor in the progression of atherosclerosis. During disease progression, SMCs experience major changes in their microenvironment, such as what integrin-binding sites are exposed, the portfolio of soluble factors available, and the elasticity and modulus of the surrounding vessel wall. We have developed a hydrogel biomaterial platform to examine the combined effect of these changes on SMC phenotype. We were particularly interested in how the chemical microenvironment affected the ability of SMCs to sense and respond to modulus. To our surprise, we observed that integrin binding and soluble factors are major drivers of several critical SMC behaviors, such as motility, proliferation, invasion, and differentiation marker expression, and these factors modulated the effect of stiffness on proliferation and migration. Overall, modulus only modestly affected behaviors other than proliferation, relative to integrin binding and soluble factors. Surprisingly, pathological behaviors (proliferation, motility) are not inversely related to SMC marker expression, in direct conflict with previous studies on substrates coupled with single extracellular matrix (ECM) proteins. A high-throughput bead-based ELISA approach and inhibitor studies revealed that differentiation marker expression is mediated chiefly via focal adhesion kinase (FAK) signaling, and we propose that integrin binding and FAK drive the transition from a migratory to a proliferative phenotype. We emphasize the importance of increasing the complexity of in vitro testing platforms to capture these subtleties in cell phenotypes and signaling, in order to better recapitulate important features of in vivo disease and elucidate potential context-dependent therapeutic targets.






Similar content being viewed by others
References
Abou Zeid, N., A.-M. Vallés, and B. Boyer. Serine phosphorylation regulates paxillin turnover during cell migration. Cell Commun. Signal. 4:8, 2006.
Anseth, K. S., C. N. Bowman, and L. Brannon-Peppas. Mechanical properties of hydrogels and their experimental determination. Biomaterials 17(17):1647–1657, 1996.
Arias-Salgado, E. G., S. Lizano, S. Sarkar, J. S. Brugge, M. H. Ginsberg, and S. J. Shattil. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl. Acad. Sci. USA 100(23):13298–13302, 2003.
Bellis, S., J. Perrotta, M. Curtis, and C. Turner. Adhesion of fibroblasts to fibronectin stimulates both serine and tyrosine phosphorylation of paxillin. Biochem. J. 381:375–381, 1997.
Bjorkerud, S. Effects of transforming growth factor-beta 1 on human arterial smooth muscle cells in vitro. Arterioscler. Thromb. Vasc. Biol. 11(4):892–902, 1991.
Blank, R. S., and G. K. Owens. Platelet-derived growth factor regulates actin isoform expression and growth state in cultured rat aortic smooth muscle cells. J. Cell. Physiol. 142(3):635–642, 1990.
Bornfeldt, K. E. Intracellular signaling in arterial smooth muscle migration versus proliferation. Trends Cardiovasc. Med. 6(5):143–151, 1996.
Castellot, J. J., L. A. Pukac, B. L. Caleb, T. C. Wright, and M. J. Karnovsky. Heparin selectively inhibits a protein kinase C-dependent mechanism of cell cycle progression in calf aortic smooth muscle cells. J. Cell Biol. 109(6):3147–3155, 1989.
Corjay, M. H., M. M. Thompson, K. R. Lynch, and G. K. Owens. Differential effect of platelet-derived growth factor-versus serum-induced growth on smooth muscle alpha-actin and nonmuscle beta-actin mRNA expression in cultured rat aortic smooth muscle cells. J. Biol. Chem. 264(18):10501–10506, 1989.
De Donatis, A., G. Comito, F. Buricchi, M. C. Vinci, A. Parenti, A. Caselli, G. Camici, G. Manao, G. Ramponi, and P. Cirri. Proliferation versus migration in platelet-derived growth factor signaling: the key role of endocytosis. J. Biol. Chem. 283(29):19948–19956, 2008.
De Donatis, A., F. Ranaldi, and P. Cirri. Reciprocal control of cell proliferation and migration. Cell Commun. Signal. 8:20, 2010.
Desmouliere, A., L. Rubbia-Brandt, and G. Gabbiani. Modulation of actin isoform expression in cultured arterial smooth muscle cells by heparin and culture conditions. Arterioscler. Thromb. Vasc. Biol. 11(2):244–253, 1991.
Engler, A., L. Bacakova, C. Newman, A. Hategan, M. Griffin, and D. Discher. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 86(1 Pt 1):617–628, 2004.
Gaudet, C., W. A. Marganski, S. Kim, C. T. Brown, V. Gunderia, M. Dembo, and J. Y. Wong. Influence of type I collagen surface density on fibroblast spreading, motility, and contractility. Biophys. J. 85(5):3329–3335, 2003.
Hadjipanayi, E., V. Mudera, and R. A. Brown. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J. Tissue Eng. Regen. Med. 3(2):77–84, 2009.
Hautmann, M. B., C. S. Madsen, and G. K. Owens. A transforming growth factor beta (TGFbeta) control element drives TGFbeta-induced stimulation of smooth muscle alpha-actin gene expression in concert with two CArG elements. J. Biol. Chem. 272(16):10948–10956, 1997.
Hazeltine, L. B., C. S. Simmons, M. R. Salick, X. Lian, M. G. Badur, W. Han, S. M. Delgado, T. Wakatsuki, W. C. Crone, B. L. Pruitt, and S. P. Palecek. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int. J. Cell Biol. 2012:508294, 2012.
Herrick, W. G., T. V. Nguyen, M. Sleiman, S. McRae, T. S. Emrick, and S. R. Peyton. PEG-phosphorylcholine hydrogels as tunable and versatile platforms for mechanobiology. Biomacromolecules 14(7):2294–2304, 2013.
Hollenbeck, S. T., H. Itoh, O. Louie, P. L. Faries, B. Liu, and K. C. Kent. Type I collagen synergistically enhances PDGF-induced smooth muscle cell proliferation through pp60src-dependent crosstalk between the alpha2beta1 integrin and PDGF beta receptor. Biochem. Biophys. Res. Commun. 325(1):328–337, 2004.
Holycross, B. J., R. S. Blank, M. M. Thompson, M. J. Peach, and G. K. Owens. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ. Res. 71(6):1525–1532, 1992.
Ishigaki, T., K. Imanaka-Yoshida, N. Shimojo, S. Matsushima, W. Taki, and T. Yoshida. Tenascin-C enhances crosstalk signaling of integrin αvβ3/PDGFR-β complex by SRC recruitment promoting PDGF-induced proliferation and migration in smooth muscle cells. J. Cell. Physiol. 226(10):2617–2624, 2011.
Janat, M. F., W. S. Argraves, and G. Liau. Regulation of vascular smooth muscle cell integrin expression by transforming growth factor beta1 and by platelet-derived growth factor-BB. J. Cell. Physiol. 151(3):588–595, 1992.
Jones, J. I., T. Prevette, A. Gockerman, and D. R. Clemmons. Ligand occupancy of the alpha-V-beta3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor. Proc. Natl. Acad. Sci. USA 93(6):2482–2487, 1996.
Li, S., S. Sims, Y. Jiao, L. H. Chow, and J. G. Pickering. Evidence from a novel human cell clone that adult vascular smooth muscle cells can convert reversibly between noncontractile and contractile phenotypes. Circ. Res. 85(4):338–348, 1999.
Lindner, V., N. E. Olson, A. W. Clowes, and M. A. Reidy. Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. J. Clin. Invest. 90(5):2044–2049, 1992.
Louis, S. F., and P. Zahradka. Vascular smooth muscle cell motility: from migration to invasion. Exp. Clin. Cardiol. 15(4):e75–e85, 2010.
Macri, L., D. Silverstein, and R. A. F. Clark. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv. Drug Deliv. Rev. 59(13):1366–1381, 2007.
Mann, B., and J. West. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J. Biomed. Mater. Res. 60(1):86–93, 2002.
McCaffrey, T. A., D. J. Falcone, C. F. Brayton, L. A. Agarwal, F. G. Welt, and B. B. Weksler. Transforming growth factor-beta activity is potentiated by heparin via dissociation of the transforming growth factor-beta/alpha 2-macroglobulin inactive complex. J. Cell Biol. 109(1):441–448, 1989.
Mitra, S. K., and D. D. Schlaepfer. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18(5):516–523, 2006.
Nelson, P. R., S. Yamamura, and K. C. Kent. Extracellular matrix proteins are potent agonists of human smooth muscle cell migration. J. Vasc. Surg. 24(1):25–32, 1996.
Nguyen, T. V., M. Sleiman, T. Moriarty, W. G. Herrick, and S. R. Peyton. Sorafenib resistance and JNK signaling in carcinoma during extracellular matrix stiffening. Biomaterials 35(22):5749–5759, 2014.
Peyton, S. R., P. D. Kim, C. M. Ghajar, D. Seliktar, and A. J. Putnam. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials 29(17):2597–2607, 2008.
Peyton, S. R., and A. J. Putnam. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J. Cell. Physiol. 204(1):198–209, 2005.
Peyton, S. R., C. B. Raub, V. P. Keschrumrus, and A. J. Putnam. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials 27(28):4881–4893, 2006.
Pirone, D. M., W. F. Liu, S. A. Ruiz, L. Gao, S. Raghavan, C. A. Lemmon, L. H. Romer, and C. S. Chen. An inhibitory role for FAK in regulating proliferation: a link between limited adhesion and RhoA-ROCK signaling. J. Cell Biol. 174(2):277–288, 2006.
Rensen, S. S. M., P. A. F. M. Doevendans, and G. J. J. M. van Eys. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 15(3):100–108, 2007.
Sazonova, O. V., B. C. Isenberg, J. Herrmann, K. L. Lee, A. Purwada, A. D. Valentine, J. A. Buczek-Thomas, J. Y. Wong, and M. A. Nugent. Extracellular matrix presentation modulates vascular smooth muscle cell mechanotransduction. Matrix Biol. 41:36–43, 2015.
Shi, Z. D., G. Abraham, and J. M. Tarbell. Shear stress modulation of smooth muscle cell marker genes in 2-D and 3-D depends on mechanotransduction by heparan sulfate proteoglycans and ERK1/2. PLoS One 5(8):e12196, 2010.
Shull, K. R., D. Ahn, W.-L. Chen, C. M. Flanigan, and A. J. Crosby. Axisymmetric adhesion tests of soft materials. Macromol. Chem. Phys. 199(4):489–511, 1998.
Skaletz-Rorowski, A., A. Schmidt, G. Breithardt, and E. Buddecke. Heparin-induced overexpression of basic fibroblast growth factor, basic fibroblast growth factor receptor, and cell-associated proteoheparan sulfate in cultured coronary smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 16(8):1063–1069, 1996.
Stegemann, J. P., and R. M. Nerem. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three-dimensional culture. Exp. Cell Res. 283(2):146–155, 2003.
Stephan, S., S. G. Ball, M. Williamson, D. V. Bax, A. Lomas, C. A. Shuttleworth, and C. M. Kielty. Cell-matrix biology in vascular tissue engineering. J. Anat. 209(4):495–502, 2006.
Ucuzian, A. A., L. P. Brewster, A. T. East, Y. Pang, A. A. Gassman, and H. P. Greisler. Characterization of the chemotactic and mitogenic response of SMCs to PDGF-BB and FGF-2 in fibrin hydrogels. J. Biomed. Mater. Res. Part A 94(3):988–996, 2010.
van Eys, G. J., P. M. Niessen, and S. S. Rensen. Smoothelin in vascular smooth muscle cells. Trends Cardiovasc. Med. 17(1):26–30, 2007.
Winder, S. J., B. G. Allen, E. D. Fraser, H. M. Kang, G. J. Kargacin, and M. P. Walsh. Calponin phosphorylation in vitro and in intact muscle. Biochem. J. 296:827–836, 1993.
Wong, J. Y., A. Velasco, P. Rajagopalan, and Q. Pham. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir 19(9):1908–1913, 2003.
Worth, N. F., B. E. Rolfe, J. Song, and G. R. Campbell. Vascular smooth muscle cell phenotypic modulation in culture is associated with reorganisation of contractile and cytoskeletal proteins. Cell Motil. Cytoskelet. 49(3):130–145, 2001.
Zheng, B., C. Duan, and D. R. Clemmons. The effect of extracellular matrix proteins on porcine smooth muscle cell insulin-like growth factor (IGF) binding protein-5 synthesis and responsiveness to IGF-I. J. Biol. Chem. 273:8994–9000, 1998.
Acknowledgements
This work was supported by a Grant in Aid from the American Heart Association (13GRNT16190013), a Barry and Afsaneh Siadat Career Development Award, grants from the National Science Foundation and the National Cancer Institute (DMR-1234852 and DMR-1304724), start-up funds from the University of Massachusetts Amherst, and the UMass MRSEC on Polymers (DMR-0820506). WGH was supported by a fellowship from the Institute of Cellular Engineering IGERT at UMass (DGE-0654128). SRP is a Pew Biomedical Scholar supported by the Pew Charitable Trusts. We are also grateful to Dr. Nele Van Dessel for helpful discussions.
Conflict of interests
William G. Herrick, Shruti Rattan, Thuy V. Nguyen, Michael S. Grunwald, Christopher W. Barney, Alfred J. Crosby, and Shelly R. Peyton declare no conflicts of interest. No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Cynthia A. Reinhart-King oversaw the review of this article.
This article is part of the 2015 Young Innovators Issue.
Shelly R. Peyton is the Barry and Afsaneh Siadat Assistant Professor of Chemical Engineering at the University of Massachusetts, Amherst. She received her B.S. in Chemical Engineering from Northwestern University in 2002 and went on to obtain her MS and Ph.D. in Chemical Engineering from the University of California, Irvine. She was then an NIH Kirschstein post-doctoral fellow in the Biological Engineering department at MIT before starting her academic appointment at UMass in 2011. Her research interests are in biomaterial design and understanding how cell-material interactions contribute to cancer aggressiveness, cardiovascular disease progression, and regenerative medicine. Since arriving at UMass she has been named a Pew Biomedical Scholar, received a New Innovator Award from the NIH, and she was recently awarded a CAREER grant from the NSF.

Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herrick, W.G., Rattan, S., Nguyen, T.V. et al. Smooth Muscle Stiffness Sensitivity is Driven by Soluble and Insoluble ECM Chemistry. Cel. Mol. Bioeng. 8, 333–348 (2015). https://doi.org/10.1007/s12195-015-0397-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-015-0397-4