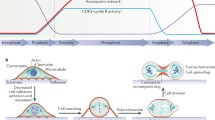
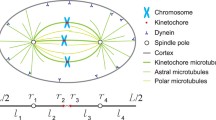
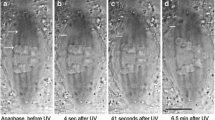
Abstract
When chromosomes are being separated in preparation for cell division, their motions are slow (~16 nm/s) relative to the speed at which many motor enzymes can move their cellular cargoes (160–1000 nm/s and sometimes even faster) and at which microtubules depolymerize (∼200 nm/s). Indeed, anaphase chromosome speeds are so slow that viscous drag puts little load on the mechanisms that generate the relevant forces (Nicklas, Adv. Cell Biol. 2:225, 1971). Available evidence suggests that chromosome speed is due to some form of regulation. For example, big and little chromosomes move at about the same speed, chromosomes that have farther to go move faster than others, and chromosome speed is affected by both temperature and an experimentally applied load. In this essay we review data on these phenomena and present our ideas about likely properties of the mechanisms that regulate chromosome speed.






Similar content being viewed by others
References
Aist, J. R., C. J. Bayles, W. Tao, and M. W. Berns. Direct experimental evidence for the existence, structural basis and function of astral forces during anaphase b in vivo. J. Cell Sci. 100(2):279–288, 1991. http://jcs.biologists.org/content/100/2/279.
Akiyoshi, B., K. K. Sarangapani, A. F. Powers, C. R. Nelson, S. L. Reichow, H. Arellano-Santoyo, T. Gonen, J. A. Ranish, C. L. Asbury, and S. Biggins. Tension directly stabilizes reconstituted kinetochore–microtubule attachments. Nature 468(7323):576–579, 2010. http://www.nature.com/nature/journal/v468/n7323/full/nature09594.html.
Alushin, G. M., V. H. Ramey, S. Pasqualato, D. A. Ball, N. Grigorieff, A. Musacchio, and E. Nogales. The ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature 467(7317):805–810, 2010. http://www.nature.com/nature/journal/v467/n7317/abs/nature09423.html.
Betterton, M. D., and F. Jülicher. Opening of nucleic-acid double strands by helicases: active versus passive opening. Phys. Rev. E 71(1): 011904, 2005. http://link.aps.org/doi/10.1103/PhysRevE.71.011904.
Bormuth, V., V. Varga, J. Howard, and E. Schaffer. Protein friction limits diffusive and directed movements of kinesin motors on microtubules. Science 325(5942):870–873, 2009. http://www.sciencemag.org/cgi/doi/10.1126/science.1174923.
Brouhard, G. J., and A. J. Hunt. Microtubule movements on the arms of mitotic chromosomes: polar ejection forces quantified in vitro. Proc. Natl Acad. Sci. U.S.A. 102(39):13903–13908, 2005. http://www.pnas.org/content/102/39/13903.
Brust-Mascher, I., and J. M. Scholey. Mitotic spindle dynamics in Drosophila. In: International Review of Cytology, Vol. 259, edited by K. W. Jeon. San Diego: Academic Press, 2007, pp. 139–172. http://www.sciencedirect.com/science/article/pii/S0074769606590047.
Cheeseman, I. M., and A. Desai. Molecular architecture of the kinetochore–microtubule interface. Nat. Rev. Mol. Cell Biol. 9(1):33–46, 2008. http://www.nature.com/nrm/journal/v9/n1/full/nrm2310.html.
Chen, W., and D. Zhang. Kinetochore fibre dynamics outside the context of the spindle during anaphase. Nat. Cell Biol. 6(3):227–231, 2004. http://www.nature.com/ncb/journal/v6/n3/abs/ncb1104.html.
Daniels, B. R., B. C. Masi, and D. Wirtz. Probing single-cell micromechanics in vivo: the microrheology of C. elegans developing embryos. Biophys. J. 90(12):4712–4719, 2006. http://www.sciencedirect.com/science/article/pii/S0006349506726467.
DeLuca, J. G., and A. Musacchio. Structural organization of the kinetochore–microtubule interface. Curr. Opin. Cell Biol. 24(1):48–56, 2012. http://www.sciencedirect.com/science/article/pii/S0955067411001499.
Desai, A., P. S. Maddox, T. J. Mitchison, and E. D. Salmon. Anaphase a chromosome movement and poleward spindle microtubule flux occur at similar rates in xenopus extract spindles. J. Cell Biol. 141(3):703–713, 1998. http://jcb.rupress.org/content/141/3/703.
Desai, A., and T. J. Mitchison. A new role for motor proteins as couplers to depolymerizing microtubules. J. Cell Biol. 128(1/2):1–4, 1995. http://www.jstor.org/stable/1616720.
Doi, M., and S. F. Edwards. The Theory of Polymer Dynamics, Vol. 73. New York: Oxford University Press, 1988.
Dumont, S., and T. Mitchison. Mechanical forces in mitosis. In: Comprehensive Biophysics, Vol. 4, edited by E. Egelman. Amsterdam: Elsevier.
Ferraro-Gideon, J., R. Sheykhani, Q. Zhu, M. L. Duquette, M. W. Berns, and A. Forer. Measurements of forces produced by the mitotic spindle using optical tweezers. Mol. Biol. Cell. 24(9):1375–1386. http://www.molbiolcell.org/content/early/2013/03/11/mbc.E12-12-0901.
Gorbsky, G. J., P. J. Sammak, and G. G. Borisy. Chromosomes move poleward in anaphase along stationary microtubules that coordinately disassemble from their kinetochore ends. J. Cell Biol. 104(1):9–18, 1987. http://jcb.rupress.org/content/104/1/9.
Grishchuk, E. L., and J. R. McIntosh. Microtubule depolymerization can drive poleward chromosome motion in fission yeast. EMBO J. 25(20):4888–4896, 2006.
Grishchuk, E. L., I. S. Spiridonov, V. A. Volkov, A. Efremov, S. Westermann, D. Drubin, G. Barnes, F. I. Ataullakhanov, and J. R. McIntosh. Different assemblies of the DAM1 complex follow shortening microtubules by distinct mechanisms. Proc. Natl Acad. Sci. U.S.A. 105(19):6918–6923, 2008. http://www.pnas.org/content/105/19/6918.
Gupta, M. L., P. Carvalho, D. M. Roof, and D. Pellman. Plus end-specific depolymerase activity of kip3, a kinesin-8 protein, explains its role in positioning the yeast mitotic spindle. Nat. Cell Biol. 8(9):913–923, 2006. http://www.nature.com/ncb/journal/v8/n9/full/ncb1457.html.
Labbé, J., E. K. McCarthy, and B. Goldstein. The forces that position a mitotic spindle asymmetrically are tethered until after the time of spindle assembly. J. Cell Biol. 167(2):245–256, 2004. http://jcb.rupress.org/content/167/2/245.
LaFountain, J. R., C. S. Cohan, A. J. Siegel, and D. J. LaFountain. Direct visualization of microtubule flux during metaphase and anaphase in Crane-Fly spermatocytes. Mol. Biol. Cell 15(12):5724–5732, 2004. http://www.molbiolcell.org/content/15/12/5724.
Maddox, P., A. Desai, K. Oegema, T. J. Mitchison, and E. Salmon. Poleward microtubule flux is a major component of spindle dynamics and anaphase a in mitotic Drosophila embryos. Curr. Biol. 12(19):1670–1674, 2002. http://www.sciencedirect.com/science/article/pii/S0960982202011831.
Maddox, P., A. Straight, P. Coughlin, T. J. Mitchison, and E. D. Salmon. Direct observation of microtubule dynamics at kinetochores in xenopus extract spindles implications for spindle mechanics. J. Cell Biol. 162(3):377–382, 2003. http://jcb.rupress.org/content/162/3/377.
Maddox, P. S., K. S. Bloom, and E. D. Salmon. The polarity and dynamics of microtubule assembly in the budding yeast saccharomyces cerevisiae. Nat. Cell Biol. 2(1):36, 2000. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2879060/.
Magidson, V., C. B. O’Connell, J. Lončarek, R. Paul, A. Mogilner, and A. Khodjakov. The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell 146(4):555–567, 2011. http://www.sciencedirect.com/science/article/pii/S0092867411007732.
McIntosh, J. R., E. L. Grishchuk, M. K. Morphew, A. K. Efremov, K. Zhudenkov, V. A. Volkov, I. M. Cheeseman, A. Desai, D. N. Mastronarde, and F. I. Ataullakhanov. Fibrils connect microtubule tips with kinetochores: a mechanism to couple tubulin dynamics to chromosome motion. Cell 135(2):322–333, 2008. http://www.sciencedirect.com/science/article/pii/S0092867408011197.
Mcintosh, J. R., P. K. Hepler, and D. G. V. Wie. Model for mitosis. Nature 224(5220):659–663, 1969. http://www.nature.com/nature/journal/v224/n5220/abs/224659a0.html.
McIntosh, J. R., M. I. Molodtsov, and F. I. Ataullakhanov. Biophysics of mitosis. Q. Rev. Biophys. 45(2):147–207, 2012.
McIntosh, J. R., E. O’Toole, K. Zhudenkov, M. Morphew, C. Schwartz, F. I. Ataullakhanov, and E. L. Grishchuk. Conserved and divergent features of kinetochores and spindle microtubule ends from five species. J. Cell Biol. 200(4):459–474, 2013. http://jcb.rupress.org/content/200/4/459.
McIntosh, J. R., V. Volkov, F. I. Ataullakhanov, and E. L. Grishchuk. Tubulin depolymerization may be an ancient biological motor. J. Cell Sci. 123(20):3425–3434, 2010. http://jcs.biologists.org/content/123/20/3425.
Miranda, J. L., P. D. Wulf, P. K. Sorger, and S. C. Harrison. The yeast DASH complex forms closed rings on microtubules. Nat. Struct. Mol. Biol. 12(2):138–143, 2005. http://www.nature.com/nsmb/journal/v12/n2/abs/nsmb896.html.
Mitchison, T. J. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J. Cell Biol. 109(2):637–652, 1989. http://jcb.rupress.org/content/109/2/637.
Nicklas, R. B. Chromosome velocity during mitosis as a function of chromosome size and position. J. Cell Biol. 25(1):19–135, 1965. http://jcb.rupress.org/content/25/1/119.
Nicklas, R. B. Mitosis. Adv. Cell Biol. 2:225, 1971. http://europepmc.org/abstract/MED/5005502.
Nicklas, R. B. Chromosome movement and spindle birefringence in locally heated cells: interaction versus local control. Chromosoma 74(1):1–37, 1979. http://link.springer.com/article/10.1007/BF00344480.
Nicklas, R. B. Measurements of the force produced by the mitotic spindle in anaphase. J. Cell Biol. 97(2):542–548, 1983. http://jcb.rupress.org/content/97/2/542.
Nicklas, R. B., and G. W. Gordon. The total length of spindle microtubules depends on the number of chromosomes present. J. Cell Biol. 100(1):1–7, 1985. http://jcb.rupress.org/content/100/1/1.
Nicklas, R. B., G. M. Lee, C. L. Rieder, and G. Rupp. Mechanically cut mitotic spindles: clean cuts and stable microtubules. J. Cell Sci. 94(3):415–423, 1989. http://jcs.biologists.org/content/94/3/415.
Rebhun, L. I. Structural aspects of saltatory particle movement. J. Gen. Physiol. 50(6):223–239, 1967. http://jgp.rupress.org/content/50/6/223.
Rieder, C. L., E. A. Davison, L. C. Jensen, L. Cassimeris, and E. D. Salmon. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and half-spindle. J. Cell Biol. 103(2):581–591, 1986. http://jcb.rupress.org/content/103/2/581.
Rogers, G. C., S. L. Rogers, T. A. Schwimmer, S. C. Ems-McClung, C. E. Walczak, R. D. Vale, J. M. Scholey, and D. J. Sharp. Two mitotic kinesins cooperate to drive sister chromatid separation during anaphase. Nature 427(6972):364–370, 2004. http://www.nature.com/nature/journal/v427/n6972/abs/nature02256.html.
Roos, U. Light and electron microscopy of rat kangaroo cells in mitosis. Chromosoma 40(1):43–82, 1973. http://link.springer.com/article/10.1007/BF00319836.
Roos, U. Light and electron microscopy of rat kangaroo cells in mitosis. Chromosoma 54(4):363–385, 1976. http://europepmc.org/abstract/MED/1253643.
Ruth, F., R. Sommese, J. Sung, S. Nag, S. Sutton, J. Deacon, E. Choe, L. Leinwand K. Ruppel, and J. Spudich. Molecular consequences of the R453C hypertrophic cardiomyopathy mutation on human β-cardiac myosin motor function. Proc. Natl Acad. Sci. U.S.A. 110(31):12607–12612.
Saunders, A. M., J. Powers, S. Strome, and W. M. Saxton. Kinesin-5 acts as a brake in anaphase spindle elongation. Curr. Biol. 17(12):R453–R454, 2007. http://www.sciencedirect.com/science/article/pii/S0960982207013334.
Saxton, W. M., and J. R. McIntosh. Interzone microtubule behavior in late anaphase and telophase spindles. J. Cell Biol. 105(2):875–886, 1987. http://jcb.rupress.org/content/105/2/875.
Schaap, C. J., and A. Forer. Temperature effects on anaphase chromosome movement in the spermatocytes of two species of crane flies (Nephrotoma suturalis loew and Nephrotoma ferruginea fabricius). J. Cell Sci. 39(1):29–52, 1979. http://jcs.biologists.org/content/39/1/29.
Schmidt, J. C., H. Arthanari, A. Boeszoermenyi, N. M. Dashkevich, E. M. Wilson-Kubalek, N. Monnier, M. Markus, M. Oberer, R. A. Milligan, M. Bathe, G. Wagner, E. L. Grishchuk, and I. M. Cheeseman. The Kinetochore-Bound ska1 complex tracks depolymerizing microtubules and binds to curved protofilaments. Dev. Cell 23(5):968–980, 2012. http://www.sciencedirect.com/science/article/pii/S1534580712004236.
Skibbens, R. V., V. P. Skeen, and E. D. Salmon. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push-pull mechanism. J. Cell Biol. 122(4):859–875, 1993. URL http://jcb.rupress.org/content/122/4/859.
Spurck, T. P., and J. D. Pickett-Heaps. On the mechanism of anaphase a: evidence that ATP is needed for microtubule disassembly and not generation of polewards force. J. Cell Biol. 105(4):1691–1705, 1987. http://jcb.rupress.org/content/105/4/1691.
Stumpff, J., and L. Wordeman. Chromosome congression: the Kinesin-8-Step path to alignment. Curr. Biol. 17(9):R326–R328, 2007. http://www.sciencedirect.com/science/article/pii/S0960982207011141.
Tanaka, K., E. Kitamura, Y. Kitamura, and T. U. Tanaka. Molecular mechanisms of microtubule-dependent kinetochore transport toward spindle poles. J. Cell Biol. 178(2):269–281, 2007. http://jcb.rupress.org/content/178/2/269.
Taylor, E. W. Some comments on the mechanism of mitosis. Soc. Gen. Physiol. 30:1, 1975. http://www.ncbi.nlm.nih.gov/pubmed/1188414.
Varga, V., C. Leduc, V. Bormuth, S. Diez, and J. Howard. Kinesin-8 motors act cooperatively to mediate length-dependent microtubule depolymerization. Cell 138(6):1174–1183, 2009. http://linkinghub.elsevier.com/retrieve/pii/S009286740900912X.
Volkov, V. A., A. V. Zaytsev, N. Gudimchuk, P. M. Grissom, A. L. Gintsburg, F. I. Ataullakhanov, J. R. McIntosh, and E. L. Grishchuk. Long tethers provide high-force coupling of the dam1 ring to shortening microtubules. Proc. Natl. Acad. Sci. U.S.A. 110(19):7708–7713, 2013. http://www.pnas.org/content/early/2013/04/18/1305821110.
Wang, S. Z., and R. Adler. Chromokinesin: a DNA-binding, kinesin-like nuclear protein. J. Cell Biol. 128(5):761–768, 1995. http://jcb.rupress.org/content/128/5/761.
West, R. R., T. Malmstrom, and J. R. McIntosh. Kinesins klp5+ and klp6+ are required for normal chromosome movement in mitosis. J. Cell Sci. 115(5):931–940, 2002. http://jcs.biologists.org/content/115/5/931.short.
Westermann, S., H. Wang, A. Avila-Sakar, D. G. Drubin, E. Nogales, and G. Barnes. The dam1 kinetochore ring complex moves processively on depolymerizing microtubule ends. Nature 440(7083):565–569, 2006. http://www.nature.com/nature/journal/v440/n7083/full/nature04409.html.
Yang, G., L. A. Cameron, P. S. Maddox, E. D. Salmon, and G. Danuser. Regional variation of microtubule flux reveals microtubule organization in the metaphase meiotic spindle. J. Cell Biol. 182(4):631–639, 2008. http://jcb.rupress.org/content/182/4/631.
Acknowledgments
The authors thank Loren Hough, Fazly Ataullakhanov and members of their labs for helpful discussions. This work was supported by NSF CAREER Award DMR-0847685 to MDB and GM033587 to JRM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor David Odde oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Betterton, M.D., McIntosh, J.R. Regulation of Chromosome Speeds in Mitosis. Cel. Mol. Bioeng. 6, 418–430 (2013). https://doi.org/10.1007/s12195-013-0297-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-013-0297-4