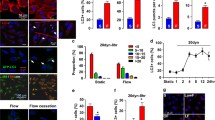
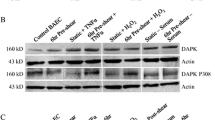
Abstract
As the interface between circulation and the vessel wall, the endothelium dynamically responds to shear-stress imposed by blood flow, which is essential to maintain vascular homeostasis. Results from in vitro studies of cultured vascular endothelial cells and in vivo examination of animal models suggest that adenosine monophosphate-activated protein kinase (AMPK), a master regulator of energy metabolism, is activated by shear stress. Thus, beyond its role in orchestrating cellular energy balance, shear-stress-activated AMPK is crucial for maintaining endothelial homeostasis by its regulation of key molecules such as endothelial nitric oxide synthase (eNOS), Kruppel-like factor 2 (KLF2), and mammalian target of rapamycin (mTOR). Collectively, shear-stress-activated AMPK enhances nitric oxide bioavailability, arrests the cell cycle, and upregulates anti-oxidative and anti-inflammatory genes. These regulations contribute to the atheroprotective effects. This review summarizes some of the recent findings of these mechanotransduction mechanisms, which indicate that AMPK plays an important role in vascular homeostasis in addition to its involvement in energy metabolism.


Similar content being viewed by others
References
Akimoto, S., M. Mitsumata, T. Sasaguri, and Y. Yoshida. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1). Circ. Res. 86:185–190, 2000.
Alba, G., R. El Bekay, M. Alvarez-Maqueda, P. Chacon, A. Vega, J. Monteseirin, C. Santa Maria, E. Pintado, F. J. Bedoya, R. Bartrons, and F. Sobrino. Stimulators of AMP-activated protein kinase inhibit the respiratory burst in human neutrophils. FEBS Lett. 573:219–225, 2004.
Amodeo, G. A., M. J. Rudolph, and L. Tong. Crystal structure of the heterotrimer core of Saccharomyces cerevisiae AMPK homologue SNF1. Nature. 449:492–495, 2007.
Andersson, U., K. Filipsson, C. R. Abbott, A. Woods, K. Smith, S. R. Bloom, D. Carling, and C. J. Small. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 279:12005–12008, 2004.
Bateman, A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem. Sci. 22:12–13, 1997.
Blackwell, K. A., J. P. Sorenson, D. M. Richardson, L. A. Smith, O. Suda, K. Nath, and Z. S. Katusic. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am. J. Physiol. Heart Circ. Physiol. 287:H2448–H2453, 2004.
Boo, Y. C., G. Sorescu, N. Boyd, I. Shiojima, K. Walsh, J. Du, and H. Jo. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J. Biol. Chem. 277:3388–3396, 2002.
Boo, Y. C., G. P. Sorescu, P. M. Bauer, D. Fulton, B. E. Kemp, D. G. Harrison, W. C. Sessa, and H. Jo. Endothelial NO synthase phosphorylated at SER635 produces NO without requiring intracellular calcium increase. Free Radic. Biol. Med. 35:729–741, 2003.
Browne, G. J., S. G. Finn, and C. G. Proud. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J. Biol. Chem. 279:12220–12231, 2004.
Brunn, G. J., C. C. Hudson, A. Sekulic, J. M. Williams, H. Hosoi, P. J. Houghton, J. C. Lawrence, Jr., and R. T. Abraham. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277:99–101, 1997.
Carling, D., P. R. Clarke, V. A. Zammit, and D. G. Hardie. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur. J. Biochem. 186:129–136, 1989.
Caro, C. G. Discovery of the role of wall shear in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29:158–161, 2009.
Ceolotto, G., A. Gallo, I. Papparella, L. Franco, E. Murphy, E. Iori, E. Pagnin, G. P. Fadini, M. Albiero, A. Semplicini, and A. Avogaro. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 27:2627–2633, 2007.
Chen, Z., I. C. Peng, X. Cui, Y. S. Li, S. Chien, and J. Y. Shyy. Shear stress, SIRT1, and vascular homeostasis. Proc. Natl Acad. Sci. USA 107:10268–10273, 2010.
Chen, Z., I. C. Peng, W. Sun, M. I. Su, P. H. Hsu, Y. Fu, Y. Zhu, K. DeFea, S. Pan, M. D. Tsai, and J. Y. Shyy. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ. Res. 104:496–505, 2009.
Chen, Z. P., K. I. Mitchelhill, B. J. Michell, D. Stapleton, I. Rodriguez-Crespo, L. A. Witters, D. A. Power, P. R. de Ortiz Montellano, and B. E. Kemp. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 443:285–289, 1999.
Chien, S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am. J. Physiol. Heart Circ. Physiol. 292:H1209–H1224, 2007.
Chiu, J. J., and S. Chien. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91:327–387, 2011.
Chiu, J. J., P. L. Lee, C. N. Chen, C. I. Lee, S. F. Chang, L. J. Chen, S. C. Lien, Y. C. Ko, S. Usami, and S. Chien. Shear stress increases ICAM-1 and decreases VCAM-1 and E-selectin expressions induced by tumor necrosis factor-[alpha] in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 24:73–79, 2004.
Davies, P. F., A. Remuzzi, E. J. Gordon, C. F. Dewey, Jr., and M. A. Gimbrone, Jr. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc. Natl Acad. Sci. USA 83:2114–2117, 1986.
De Keulenaer, G. W., D. C. Chappell, N. Ishizaka, R. M. Nerem, R. W. Alexander, and K. K. Griendling. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ. Res. 82:1094–1101, 1998.
Dimmeler, S., I. Fleming, B. Fisslthaler, C. Hermann, R. Busse, and A. M. Zeiher. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605, 1999.
Dixit, M., E. Bess, B. Fisslthaler, F. V. Hartel, T. Noll, R. Busse, and I. Fleming. Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc. Res. 77:160–168, 2008.
Dolinsky, V. W., J. S. Morton, T. Oka, I. Robillard-Frayne, M. Bagdan, G. D. Lopaschuk, C. Des Rosiers, K. Walsh, S. T. Davidge, and J. R. Dyck. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension 56:412–421, 2010.
Ewart, M. A., C. F. Kohlhaas, and I. P. Salt. Inhibition of tumor necrosis factor alpha-stimulated monocyte adhesion to human aortic endothelial cells by AMP-activated protein kinase. Arterioscler. Thromb. Vasc. Biol. 28:2255–2257, 2008.
Fisslthaler, B., and I. Fleming. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ. Res. 105:114–127, 2009.
Fisslthaler, B., I. Fleming, B. Keseru, K. Walsh, and R. Busse. Fluid shear stress and NO decrease the activity of the hydroxy-methylglutaryl coenzyme A reductase in endothelial cells via the AMP-activated protein kinase and FoxO1. Circ. Res. 100:e12–e21, 2007.
Fledderus, J. O., R. A. Boon, O. L. Volger, H. Hurttila, S. Yla-Herttuala, H. Pannekoek, A. L. Levonen, and A. J. Horrevoets. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28:1339–1346, 2008.
Fulton, D., J. P. Gratton, T. J. McCabe, J. Fontana, Y. Fujio, K. Walsh, T. F. Franke, A. Papapetropoulos, and W. C. Sessa. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399:597–601, 1999.
Garton, A. J., D. G. Campbell, D. Carling, D. G. Hardie, R. J. Colbran, and S. J. Yeaman. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur. J. Biochem. 179:249–254, 1989.
Gracia-Sancho, J., G. Villarreal, Jr., Y. Zhang, and G. Garcia-Cardena. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc. Res. 85:514–519, 2010.
Guo, D., S. Chien, and J. Y. Shyy. Regulation of endothelial cell cycle by laminar versus oscillatory flow: distinct modes of interactions of AMP-activated protein kinase and Akt pathways. Circ. Res. 100:564–571, 2007.
Gwinn, D. M., D. B. Shackelford, D. F. Egan, M. M. Mihaylova, A. Mery, D. S. Vasquez, B. E. Turk, and R. J. Shaw. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 30:214–226, 2008.
Ha, J., and K. H. Kim. Inhibition of fatty acid synthesis by expression of an acetyl-CoA carboxylase-specific ribozyme gene. Proc. Natl Acad. Sci. USA 91:9951–9955, 1994.
Hardie, D. G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8:774–785, 2007.
Hardie, D. G., and D. Carling. The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur. J. Biochem. 246:259–273, 1997.
Hawley, S. A., J. Boudeau, J. L. Reid, K. J. Mustard, L. Udd, T. P. Makela, D. R. Alessi, and D. G. Hardie. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2:28, 2003.
Hawley, S. A., D. A. Pan, K. J. Mustard, L. Ross, J. Bain, A. M. Edelman, B. G. Frenguelli, and D. G. Hardie. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2:9–19, 2005.
Hayashi, T., M. F. Hirshman, E. J. Kurth, W. W. Winder, and L. J. Goodyear. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47:1369–1373, 1998.
Jakobsen, S. N., D. G. Hardie, N. Morrice, and H. E. Tornqvist. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J. Biol. Chem. 276:46912–46916, 2001.
Kahn, B. B., T. Alquier, D. Carling, and D. G. Hardie. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1:15–25, 2005.
Kubota, N., W. Yano, T. Kubota, T. Yamauchi, S. Itoh, H. Kumagai, H. Kozono, I. Takamoto, S. Okamoto, T. Shiuchi, R. Suzuki, H. Satoh, A. Tsuchida, M. Moroi, K. Sugi, T. Noda, H. Ebinuma, Y. Ueta, T. Kondo, E. Araki, O. Ezaki, R. Nagai, K. Tobe, Y. Terauchi, K. Ueki, Y. Minokoshi, and T. Kadowaki. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 6:55–68, 2007.
Kukidome, D., T. Nishikawa, K. Sonoda, K. Imoto, K. Fujisawa, M. Yano, H. Motoshima, T. Taguchi, T. Matsumura, and E. Araki. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 55:120–127, 2006.
Leclerc, I., C. Lenzner, L. Gourdon, S. Vaulont, A. Kahn, and B. Viollet. Hepatocyte nuclear factor-4alpha involved in type 1 maturity-onset diabetes of the young is a novel target of AMP-activated protein kinase. Diabetes 50:1515–1521, 2001.
Levesque, M. J., and R. M. Nerem. The elongation and orientation of cultured endothelial cells in response to shear stress. J. Biomech. Eng. 107:341–347, 1985.
Lin, K., P. P. Hsu, B. P. Chen, S. Yuan, S. Usami, J. Y. Shyy, Y. S. Li, and S. Chien. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc. Natl Acad. Sci. USA 97:9385–9389, 2000.
McGarry, J. D., G. F. Leatherman, and D. W. Foster. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J. Biol. Chem. 253:4128–4136, 1978.
Momcilovic, M., S. P. Hong, and M. Carlson. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J. Biol. Chem. 281:25336–25343, 2006.
Niebauer, J., and J. P. Cooke. Cardiovascular effects of exercise: role of endothelial shear stress. J. Am. Coll. Cardiol. 28:1652–1660, 1996.
Parmar, K. M., H. B. Larman, G. Dai, Y. Zhang, E. T. Wang, S. N. Moorthy, J. R. Kratz, Z. Lin, M. K. Jain, M. A. Gimbrone, Jr., and G. Garcia-Cardena. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 116:49–58, 2006.
Polekhina, G., A. Gupta, B. J. van Denderen, S. C. Feil, B. E. Kemp, D. Stapleton, and M. W. Parker. Structural basis for glycogen recognition by AMP-activated protein kinase. Structure 13:1453–1462, 2005.
Russell, III, R. R., R. Bergeron, G. I. Shulman, and L. H. Young. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am. J. Physiol. 277:H643–H649, 1999.
Ruvinsky, I., and O. Meyuhas. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem. Sci. 31:342–348, 2006.
Sakamoto, K., A. McCarthy, D. Smith, K. A. Green, D. Grahame Hardie, A. Ashworth, and D. R. Alessi. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 24:1810–1820, 2005.
Sako, K., S. Fukuhara, T. Minami, T. Hamakubo, H. Song, T. Kodama, A. Fukamizu, J. S. Gutkind, G. Y. Koh, and N. Mochizuki. Angiopoietin-1 induces Kruppel-like factor 2 expression through a phosphoinositide 3-kinase/AKT-dependent activation of myocyte enhancer factor 2. J. Biol. Chem. 284:5592–5601, 2009.
Schwarz, G., G. Callewaert, G. Droogmans, and B. Nilius. Shear stress-induced calcium transients in endothelial cells from human umbilical cord veins. J. Physiol. 458:527–538, 1992.
Scott, J. W., S. A. Hawley, K. A. Green, M. Anis, G. Stewart, G. A. Scullion, D. G. Norman, and D. G. Hardie. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J. Clin. Invest. 113:274–284, 2004.
SenBanerjee, S., Z. Lin, G. B. Atkins, D. M. Greif, R. M. Rao, A. Kumar, M. W. Feinberg, Z. Chen, D. I. Simon, F. W. Luscinskas, T. M. Michel, M. A. Gimbrone, Jr., G. Garcia-Cardena, and M. K. Jain. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J. Exp. Med. 199:1305–1315, 2004.
Soucy, K. G., S. Ryoo, A. Benjo, H. K. Lim, G. Gupta, J. S. Sohi, J. Elser, M. A. Aon, D. Nyhan, A. A. Shoukas, and D. E. Berkowitz. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J. Appl. Physiol. 101:1751–1759, 2006.
Sullivan, J. E., K. J. Brocklehurst, A. E. Marley, F. Carey, D. Carling, and R. K. Beri. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 353:33–36, 1994.
Townley, R., and L. Shapiro. Crystal structures of the adenylate sensor from fission yeast AMP-activated protein kinase. Science 315:1726–1729, 2007.
Vaitkevicius, P. V., J. L. Fleg, J. H. Engel, F. C. O’Connor, J. G. Wright, L. E. Lakatta, F. C. Yin, and E. G. Lakatta. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88:1456–1462, 1993.
Van Vliet, B. N., L. L. Chafe, and J. P. Montani. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J. Physiol. 549:313–325, 2003.
Wilkinson, I. B., H. MacCallum, J. R. Cockcroft, and D. J. Webb. Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br. J. Clin. Pharmacol. 53:189–192, 2002.
Wojtaszewski, J. F., P. Nielsen, B. F. Hansen, E. A. Richter, and B. Kiens. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J. Physiol. 528(Pt. 1):221–226, 2000.
Wong, A. K., J. Howie, J. R. Petrie, and C. C. Lang. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin. Sci. (Lond.) 116:607–620, 2009.
Woods, A., K. Dickerson, R. Heath, S. P. Hong, M. Momcilovic, S. R. Johnstone, M. Carlson, and D. Carling. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2:21–33, 2005.
Xie, Z., Y. Dong, R. Scholz, D. Neumann, and M. H. Zou. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 117:952–962, 2008.
Xie, Z., Y. Dong, M. Zhang, M. Z. Cui, R. A. Cohen, U. Riek, D. Neumann, U. Schlattner, and M. H. Zou. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J. Biol. Chem. 281:6366–6375, 2006.
Xie, Z., J. Zhang, J. Wu, B. Viollet, and M. H. Zou. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes 57:3222–3230, 2008.
Xu, Q., X. Hao, Q. Yang, and L. Si. Resveratrol prevents hyperglycemia-induced endothelial dysfunction via activation of adenosine monophosphate-activated protein kinase. Biochem. Biophys. Res. Commun. 388:389–394, 2009.
Young, A., W. Wu, W. Sun, H. Benjamin Larman, N. Wang, Y. S. Li, J. Y. Shyy, S. Chien, and G. Garcia-Cardena. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler. Thromb. Vasc. Biol. 29:1902–1908, 2009.
Zhang, Y., T. S. Lee, E. M. Kolb, K. Sun, X. Lu, F. M. Sladek, G. S. Kassab, T. Garland, Jr., and J. Y. Shyy. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler. Thromb. Vasc. Biol. 26:1281–1287, 2006.
Acknowledgment
This work was supported in part by National Institutes of Health grants HL89940 and HL106579 (J.S.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Edward Guo oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Shyy, J.YJ., Chen, Z., Wu, W. et al. Shear-Stress Activation of AMP-Activated Protein Kinase in Endothelial Homeostasis. Cel. Mol. Bioeng. 4, 538–546 (2011). https://doi.org/10.1007/s12195-011-0200-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-011-0200-0