Abstract
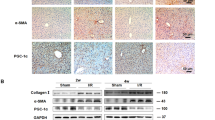
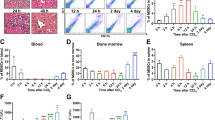
Myocardial ischemia induces cardiomyocyte injury and death, resulting in impairment of cardiac function. The adult cardiomyocytes possess a limited capacity of protection in myocardial ischemia, and nonmyocytic cells can be activated to support myocardial protection. We recently demonstrated that hepatic cells were able to upregulate genes encoding secreted proteins and were mobilized to the circulatory system, potentially contributing to myocardial protection against ischemic injury. In this investigation, we tested the potential mechanisms by which hepatic cells were mobilized in experimental myocardial ischemia. Following the induction of myocardial ischemia, hepatic cells, including hepatocytes and biliary epithelial cells, were mobilized to the circulatory system with a peak population 1.9 ± 0.4% at day 5. The cytokine IL-6 was upregulated in the ischemic myocardium as well as the serum. IL-6 promoted leukocyte retention in the liver as demonstrated by an increase in liver-retained leukocytes in myocardial ischemia in wild type mice, reduced leukocytes in IL-6−/− mice, and restoration of leukocyte retention in response to IL-6 administration to IL-6−/− mice. Liver-retained leukocytes exhibited upregulation of MMP-2, which in turn mediated hepatic cell mobilization by degrading extracellular matrix. These observations suggest that IL-6-stimulated leukocytes mediate the mobilization of hepatic cells via releasing MMP-2 in myocardial ischemia.






Similar content being viewed by others
References
Alison, M., M. Golding, El.-N. Lalani, and C. Sarraf. Wound healing in the liver with particular reference to stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353:877–894, 1998.
Angel, P. M., and M. Karin. The role of Jun, Fos, and AP-1 complex in cell proliferation and transformation. Biochim. Biophys. Acta. 1072:129–157, 1991.
Appenheimer, M. M., R. A. Girard, Q. Chen, W.-C. Wang, K. C. Bankert, J. Hardison, M. D. Bain, F. Ridgley, E. J. Sarcione, S. Buitrago, S. S. Kothlow, B. Kaspers, J. Robert, S. Rose-John, H. Baumann, and S. S. Evans. Conservation of IL-6 trans-signaling mechanisms controlling L-selectin adhesion by fever-range thermal stress. Eur. J. Immunol. 37:2856–2867, 2007.
Bergman, M. R., S. Cheng, N. Honbo, L. Piacentini, J. S. Karliner, and D. H. Lovett. A functional activating protein 1 (AP-1) site regulates matrix metalloproteinase 2 (MMP-2) transcription by cardiac cells through interactions with JunB-Fra1 and JunB-FosB heterodimers. Biochem. J. 369:485–496, 2003.
Biasucci, L. M., A. Vitelli, G. Liuzzo, S. Altamura, G. Caligiuri, C. Monaco, A. G. Rebuzzi, G. Ciliberto, and A. Maseri. Elevated levels of interleukin-6 in unstable angina. Circulation 94:874–877, 1996.
Brunner, S., J. Winogradow, B. C. Huber, M. M. Zaruba, R. Fischer, R. David, G. Assmann, N. Herbach, R. Wanke, J. Mueller-Hoecker, and W. M. Franz. Erythropoietin administration after myocardial infarction in mice attenuates ischemic cardiomyopathy associated with enhanced homing of bone marrow-derived progenitor cells via the CXCR-4/SDF-1 axis. FASEB J. 23:351–361, 2009.
Chen, Q., W.-C. Wang, R. Bruce, H. Li, D. M. Schleider, M. J. Mulbury, M. D. Bain, P. K. Wallace, H. Baumann, and S. S. Evans. Central role of IL-6 receptor signal-transducing chain gp130 in activation of L-selectin adhesion by fever-range thermal stress. Immunity 20:59–70, 2004.
Cressman, D. E., L. E. Greenbaum, R. A. DeAngelis, G. Ciliberto, E. E. Furth, V. Poli, and R. Taub. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274:1379–1383, 1996.
Dill, T., V. Schächinger, A. Rolf, S. Möllmann, H. Thiele, H. Tillmanns, B. Assmus, S. Dimmeler, A. M. Zeiher, and C. Hamm. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am. Heart J. 157:541–547, 2009.
Fausto, N., and J. S. Campbell. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech. Dev. 120:117–130, 2003.
Fazel, S. S., L. Chen, D. Angoulvant, S. H. Li, R. D. Weisel, A. Keating, and R. K. Li. Activation of c-kit is necessary for mobilization of reparative bone marrow progenitor cells in response to cardiac injury. FASEB J. 22:930–940, 2008.
Fisman, E. Z., M. Benderly, R. J. Esper, S. Behar, V. Boyko, Y. Adler, D. Tanne, Z. Matas, and A. Tenenbaum. Interleukin-6 and the risk of future cardiovascular events in patients with angina pectoris and/or healed myocardial infarction. Am. J. Cardiol. 98:14–18, 2006.
Forbes, S., P. Vig, R. Poulsom, H. Thomas, and M. Alison. Hepatic stem cells. J. Pathol. 197:510–518, 2002.
Gyöngyösi, M., I. Lang, M. Dettke, G. Beran, S. Graf, H. Sochor, N. Nyolczas, S. Charwat, R. Hemetsberger, G. Christ, I. Edes, L. Balogh, K. T. Krause, K. Jaquet, K. H. Kuck, I. Benedek, T. Hintea, R. Kiss, I. Préda, V. Kotevski, H. Pejkov, S. Zamini, A. Khorsand, G. Sodeck, A. Kaider, G. Maurer, and D. Glogar. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: the MYSTAR prospective, randomized study. Nat. Clin. Pract. Cardiovasc. Med. 6:70–81, 2009.
Heinrich, P. C., I. Behrmann, G. Muller-Newen, F. Schaper, and L. Graeve. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334:297–314, 1998.
Hirano, T., K. Yasukawa, H. Harada, T. Taga, Y. Watanabe, T. Matsuda, S. Kashiwamura, K. Nakajima, K. Koyama, and A. Iwamatsu. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 324:73–76, 1986.
Hofmann, U. B., J. R. Westphal, G. N. Van Muijen, and D. J. Ruiter. Matrix metalloproteinases in human melanoma. J. Invest. Dermatol. 115:337–344, 2000.
Ii, M., H. Nishimura, A. Iwakura, A. Wecker, E. Eaton, T. Asahara, and D. W. Losordo. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation 111:1114–1120, 2005.
Iwakura, A., S. Shastry, C. Luedemann, H. Hamada, A. Kawamoto, R. Kishore, Y. Zhu, G. Qin, M. Silver, T. Thorne, L. Eaton, H. Masuda, T. Asahara, and D. W. Losordo. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation 113:1605–1614, 2006.
Jurasz, P., G. Sawicki, M. Duszyk, J. Sawicka, C. Miranda, I. Mayers, and M. W. Radomski. Matrix metalloproteinase 2 in tumor cell-induced platelet aggregation: regulation by nitric oxide. Cancer Res. 61:376–382, 2001.
Kapoor, M., J. Martel-Pelletier, D. Lajeunesse, J. P. Pelletier, and H. Fahmi. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 7:33–42, 2011.
Kopf, M., H. Baumann, G. Freer, M. Freudenberg, M. Lamers, T. Kishimoto, R. Zinkernagel, H. Bluethmann, and G. Köhler. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339–342, 1994.
Kossakowska, A. E., D. R. Edwards, C. Prusinkiewicz, M. C. Zhang, D. Guo, S. J. Urbanski, T. Grogan, L. A. Marquez, and A. Janowska-Wieczorek. Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin’s lymphomas. Blood 94:2080–2089, 1999.
Kovalovich, K., W. Li, R. DeAngelis, L. E. Greenbaum, G. Ciliberto, and R. Taub. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J. Biol. Chem. 276:26605–26613, 2001.
Kucia, M., B. Dawn, G. Hunt, Y. Guo, M. Wysoczynski, M. Majka, J. Ratajczak, F. Rezzoug, S. T. Ildstad, R. Bolli, and M. Z. Ratajczak. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ. Res. 95:1191–1199, 2004.
Lee, J. G., S. Dahi, R. Mahimkar, N. L. Tulloch, M. A. Alfonso-Jaume, D. H. Lovett, and R. Sarkar. Intronic regulation of matrix metalloproteinase-2 revealed by in vivo transcriptional analysis in ischemia. Proc. Natl. Acad. Sci. USA. 102:16345–16350, 2005.
Liu, S. Q., P. K. Alkema, C. Tieché, B. J. Tefft, D. Z. Liu, Y. C. Li, B. E. Sumpio, J. A. Caprini, and M. Paniagua. Negative regulation of monocyte adhesion to arterial elastic laminae by signal-regulatory protein alpha and SH2 domain-containing protein tyrosine phosphatase-1. J. Biol. Chem. 280:39294–39301, 2005.
Liu, S. Q., B. J. Tefft, A. Zhang, L.-Q. Zhang, and Y. H. Wu. Formation of smooth muscle α actin filaments in CD34-positive bone marrow cells in elastic lamina-dominant matrix of arteries. Matrix Biol. 27:282–294, 2008.
Liu, S. Q., and Y. H. Wu. Cardioprotective effects of hepatic cell-derived factors in myocardial ischemia. Curr. Topics Biochem. Res. 11:65–77, 2009.
Liu, S. Q., and Y. H. Wu. Liver cell-mediated alleviation of acute ischemic myocardial injury. Front. Biosci. 2:711–724, 2010.
Lunde, K., S. Solheim, S. Aakhus, H. Arnesen, M. Abdelnoor, T. Egeland, K. Endresen, A. Ilebekk, A. Mangschau, J. G. Fjeld, H. J. Smith, E. Taraldsrud, H. K. Grøgaard, R. Bjørnerheim, M. Brekke, C. Müller, E. Hopp, A. Ragnarsson, J. E. Brinchmann, and K. Forfang. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N. Engl. J. Med. 355:1199–1209, 2006.
Malin, S. A., B. M. Davis, and D. C. Molliver. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat. Protocols 2:152–160, 2007.
Michalopoulos, G. K., and M. C. DeFrances. Liver regeneration. Science 276:60–66, 1997.
Murphy, G. Matrix metalloproteinases and their inhibitors. Acta Orthop. Scand. 266(Suppl):55–63, 1995.
Navarro-González, J. F., C. Mora-Fernández, M. M. de Fuentes, and J. García-Pérez. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011 May 3. [Epub ahead of print]
Orlic, D., J. Kajstura, S. Chimenti, F. Limana, I. Jakoniuk, F. Quaini, B. Nadal-Ginard, D. M. Bodine, A. Leri, and P. Anversa. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. PNAS 98:10344–10349, 2001.
Osypiw, J. C., R. L. Allen, and D. Billington. Subpopulations of rat hepatocytes separated by Percoll density-gradient centrifugation show characteristics consistent with different acinar locations. Biochem. J. 304:617–624, 1994.
Postic, C., M. Shiota, K. D. Niswender, T. L. Jetton, Y. Chen, J. M. Moates, K. D. Shelton, J. Lindner, A. D. Cherrington, and M. A. Magnuson. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using cre recombinase. J. Biol. Chem. 274:305–315, 1999.
Schlüter, K. D., and D. Schreiber. Adult ventricular cardiomyocytes: isolation and culture. Methods Mol. Biol. 290:305–314, 2005.
Seglen, P. O. Hepatocyte suspensions and cultures as tools in experimental carcinogenesis. J. Toxicol. Environ. Health 5:551–560, 1979.
Sehgal, P. B., A. Zilberstein, R. M. Ruggieri, L. T. May, A. Ferguson-Smith, D. L. Slate, M. Revel, and F. H. Ruddle. Human chromosome 7 carries the beta-2 interferon gene. Proc. Natl Acad. Sci. USA. 83:5219–5222, 1986.
Skinner, M., S. Qu, C. Moore, and R. Wisdom. Transcriptional activation and transformation by FosB protein require phosphorylation of the carboxyl-terminal activation domain. Mol. Cell. Biol. 17:2372–2380, 1997.
Spooren, A., K. Kolmus, G. Laureys, R. Clinckers, J. De Keyser, G. Haegeman, S. Gerlo. Interleukin-6, a mental cytokine. Brain Res. Rev. 2011 Jan 14. [Epub ahead of print]
Srinivas, S., T. Watanabe, C. S. Lin, C. M. William, Y. Tanabe, T. M. Jessell, and F. Costantini. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1:4, 2001.
Taub, R. Liver regeneration: from myth to mechanism. Nat. Rev. Mol. Cell Biol. 5:836–847, 2004.
Zar, J. H. Biostatistical Analysis. 2nd ed. Englewood Cliffs, NJ: Prentice Hall, 1984.
Acknowledgments
We would like to thank Dr. Stephen I. Levin of Northwestern University and Dr. Yan Chun Li of The University of Chicago for insightful suggestions for constructing the genetically modified mouse models. This work was supported by the National Science Foundation and American Heart Association.
Conflicts of interest
No funds in any form have been or will be received from a commercial party for the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editors John Shyy and Yingxiao Wang oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Liu, S.Q., Tefft, B.J., Zhang, B. et al. Regulation of Hepatic Cell Mobilization in Experimental Myocardial Ischemia. Cel. Mol. Bioeng. 4, 693–707 (2011). https://doi.org/10.1007/s12195-011-0197-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-011-0197-4