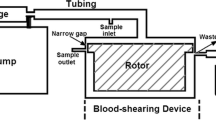
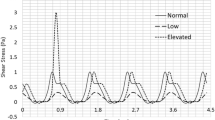
Abstract
Altered blood shear stress is one of the primary risk factors that can induce platelet activation, thrombosis, atherosclerosis, and many other cardiovascular diseases. Complement activation, an important contributor in inflammation, has also been reported to be related to blood flow-induced platelet activation. In this study, the dose effect of shear stress (magnitude and shear exposure time) on platelet surface complement activation was investigated using a dynamic cone and plate shearing device. Washed platelets were exposed to uniformly distributed shear stresses at 2.4 or 9 dyne/cm2 for 30 min. Timed samples at 0, 10, 20, and 30 min were taken and assayed for complement activation (C4d and C5b-9 deposition) using a solid-phase ELISA approach. In parallel, platelet activation was examined using flow cytometry for CD62P expression, and a modified prothrombinase assay for thrombin generation. Results indicated that activated platelets support complement activation to completion with the production of membrane attack complex C5b-9. The generation of complement activation products, C4d and C5b-9, increased significantly as shear stress magnitude and shear exposure time increased, correlating with the enhanced platelet activation levels. Therefore, platelet activation and its associated complement activation may interact and promote cardiovascular disease development under pathological shear conditions.





Similar content being viewed by others
References
Artoli AM, Sequeira A, Silva-Herdade AS, Saldanha C. Leukocytes rolling and recruitment by endothelial cells: hemorheological experiments and numerical simulations. J.Biomech. 2007;40:3493-3502.
Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock 2004;21:401-409.
Blackman BR, Barbee KA, Thibault LE. In vitro cell shearing device to investigate the dynamic response of cells in a controlled hydrodynamic environment. Ann.Biomed.Eng 2000;28:363-372.
Chatzizisis YS, Giannoglou GD. Coronary hemodynamics and atherosclerotic wall stiffness: a vicious cycle. Med.Hypotheses 2007;69:349-355.
del Conde I, Cruz MA, Zhang H, Lopez JA, fshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J.Exp.Med. 2005;201:871-879.
Enders S, Bernhard G, Zakrzewicz A, Tauber R. Inhibition of L-selectin binding by polyacrylamide-based conjugates under defined flow conditions. Biochim.Biophys.Acta 2007;1770:1441-1449.
Fung YC. Biomechanics, Circulatioin. New York: Springer; 1996.
Glantz, S. A. Primer of Biostatistics. New York: McGraw-Hill, 1997.
Gnatenko DV, Dunn JJ, McCorkle SR et al. Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood 2003;101:2285-2293.
Hellums JD. 1993 Whitaker Lecture: biorheology in thrombosis research. Ann.Biomed.Eng 1994;22:445-455.
Henson PM, Ginsberg M. Immunologic reactions of platelets. In: Gordon JL, ed. Platelets in biology and pathology. Amsterdam: Elsevier; 1981:265-308.
Jagels MA, Daffern PJ, Hugli TE. C3a and C5a enhance granulocyte adhesion to endothelial and epithelial cell monolayers: epithelial and endothelial priming is required for C3a-induced eosinophil adhesion. Immunopharmacology 2000;46:209-222.
Jesty J, Bluestein D. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Anal.Biochem. 1999;272:64-70.
Jesty J, Yin W, Perrotta P, Bluestein D. Platelet activation in a circulating flow loop: combined effects of shear stress and exposure time. Platelets. 2003;14:143-149.
Kalltorp M, Askendal A, Thomsen P, Tengvall P. Ellipsometric studies in vitro on kinetics of rat complement activation. Jounral of biomdeical materials research part A. 1999;44:222-225.
Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999;282:2035-2042.
Monsinjon T, Gasque P, Chan P et al. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003;17:1003-1014.
Peerschke EI, Ghebrehiwet B. Human blood platelets possess specific binding sites for C1q. J.Immunol. 1987;138:1537-1541.
Peerschke EI, Reid KB, Ghebrehiwet B. Identification of a novel 33-kDa C1q-binding site on human blood platelets. J.Immunol. 1994;152:5896-5901.
Peerschke EI, Yin W, Grigg SE, Ghebrehiwet B. Blood platelets activate the classical pathway of human complement. J.Thromb.Haemost. 2006;4:2035-2042.
Ridger V, Krams R, Carpi A, Evans PC. Hemodynamic parameters regulating vascular inflammation and atherosclerosis: a brief update. Biomed.Pharmacother. 2008;62:536-540.
Sims PJ, Wiedmer T. Repolarization of the membrane potential of blood platelets after complement damage: evidence for a Ca++ -dependent exocytotic elimination of C5b-9 pores. Blood 1986;68:556-561.
Wagner DD. New links between inflammation and thrombosis. Arterioscler.Thromb.Vasc.Biol. 2005;25:1321-1324.
Ware JA, Coller BS. Platelet morphology, biochemistry, and function. In: Beutler E, Lichtman MA, Coller BS, Kipps TJ, eds. Williams Hematology. New York: McGraw-Hill, Inc.; 1995:1161-1201.
Watford WT, Ghio AJ, Wright JR. Complement-mediated host defense in the lung. Am.J.Physiol.Lung Cell Mol.Physiol. 2000;279:L790-L798.
Wiedmer T, Esmon CT, Sims PJ. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood 1986;68:875-880.
Wiedmer T, Sims PJ. Effect of complement proteins C5b-9 on blood platelets. Evidence for reversible depolarization of membrane potential. J.Biol.Chem. 1985;260:8014-8019.
Wootton DM, Ku DN. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu.Rev.Biomed.Eng 1999;1:299-329.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, W., Rubenstein, D.A. Dose Effect of Shear Stress on Platelet Complement Activation in a Cone and Plate Shearing Device. Cel. Mol. Bioeng. 2, 274–280 (2009). https://doi.org/10.1007/s12195-009-0055-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-009-0055-9