Abstract
Edge methods are predominantly used for modulation transfer function (MTF) measurements in computed tomography (CT) images reconstructed using iterative methods. However, edge methods employ a relatively large and distinct test object, which is intended to simulate relatively large and distinct clinical organs. If one wants to assess the image quality of a small low-contrast object that is visually indistinct against a noisy background, a small and indistinct test object is desired. Another concern is that information related to the signal amount is discarded during MTF measurements. Choosing a weak impulse as the ultimately small test object, we have developed a tilted-wire method, which is a type of point spread function (PSF) method compatible with extremely low contrast-to-noise ratio (CNR) conditions. The signal amount is measured as the PSF volume. We used two commercial CT systems to evaluate the measurement accuracy of the tilted-wire method. When ensemble-averaged images are used, one can measure the MTF even when the wire is indiscernible from noise. The measurement error under such conditions is a few percent for both the MTF and signal amount. We also applied the tilted-wire method to two hybrid iterative reconstruction methods, namely AIDR-3D and ASiR. The results show that the MTF of ASiR is completely CNR-dependent, but that of AIDR-3D is noise-dependent. The signal amount obtained with ASiR is unchanged from that obtained through filtered back-projection (FBP). The signal amount obtained with AIDR-3D is less than that obtained through FBP, depending on the noise level.












Similar content being viewed by others
References
Richard S, Husarik DB, Yadava G, Murphy SN, Samei E. Towards task-based assessment of CT performance: system and object MTF across different reconstruction algorithms. Med Phys. 2012;39:4115–22.
Scibelli A. iDose4 iterative reconstruction technique. Philips Healthcare Whitepaper, 2011. http://acceptance.netforum.healthcare.philips.com/Clinical/global/Explore/White-Papers/CT/iDose4-iterativereconstruction-technique. Accessed 22 Feb 2018.
Chen B, Richard S, Christianson O, Zhou X, Samei E. CT performance as a variable function of resolution, noise, and task property for iterative reconstructions. Proc SPIE. 2012;8313:83131K.
Winklehner A, Karlo C, Puippe G, Schmidt B, Flohr T, Goetti R, Pfammatter T, Frauenfelder T, Alkadhi H. Raw data-based iterative reconstruction in body CTA: evaluation of radiation dose saving potential. Eur Radiol. 2011;21:2521–6.
Mori I. Non-linear Nature of Recent CT Images and Image Quality Evaluation. Bull Sch Health Sci Tohoku Univ. 2013;22:7–24.
Love A, Olsson M, Siemund R, Stålhammar F, Björkman-Burtscher M, Söderberg M. Six iterative reconstruction algorithms in brain CT: a phantom study on image quality at different radiation dose levels. Br J Radiol. 1031;2013(86):20130388.
Chen B, Christianson O, Wilson J, Samei E. Assessment of volumetric noise and resolution performance for linear and nonlinear CT reconstruction methods. Med Phys. 2014;41:071909.
Chen B, Yu L, Leng S, Kofler J, Favazza C, Vrieze T, McCollough C. Predicting detection performance with model observers: Fourier domain or spatial domain? Proc SPIE. 2016;9783:978326.
Dobeli K, Lewis S, Meikle R, Thiele D, Brenann P. Noise-reducing algorithms do not necessarily provide superior dose optimization for hepatic lesion detection with multidetector CT. Br J Radiol. 1023;2013(86):20120500.
Yu L, Leng S, Chen L, Kofler J, Carter R, McCollough C. Prediction of human observer performance in a 2-alternative forced choice low-contrast detection task using channelized Hotelling observer: impact of radiation dose and reconstruction algorithms. Med Phys. 2013;40:041908.
Mori I, Machida Y. Deriving the modulation transfer function of CT from extremely noisy edge profiles. Radiol Phys Technol. 2009;2:22–32.
Yu L, Vrieze J, Leng S, Fletcher J, McCollough C. Measuring contrast-and noise-dependent spatial resolution of an iterative reconstruction method in CT using ensemble averaging. Med Phys. 2015;42:2261–7.
Samei E, Richard S. Assessment of the dose reduction potential of a model-based iterative reconstruction algorithm using a task-based performance metrology. Med Phys. 2015;42:314–23.
Wilson J, Christianson O, Richard S, Samei E. A methodology for image quality evaluation of advanced CT systems. Med Phys. 2013;40:031908.
Friedman S, Fung G, Siewerdsen J, Tsui B. A simple approach to measure computed tomography (CT) modulation transfer function (MTF) and noise-power spectrum (NPS) using the American College of Radiology (ACR) accreditation phantom. Med Phys. 2013;40:051907.
Takata T, Ichikawa K, Mitsui W, Hayashi H, Minehiro K, Sakuta K, Nunome H, Matsubara K, Kawashima H, Matsuura Y, Gabata T. Object shape dependency of in-plane resolution for iterative reconstruction of computed tomography. Phys Med. 2017;33:146–51.
Loo L, Doi K, Metz C. A comparison of physical image quality indices and observer performance in the radiographic detection of nylon beads. Phys Med Biol. 1984;29:837–56.
Boedeker K, McNitt-Gray M. Application of the noise power spectrum in modern diagnostic MDCT: part II. Noise power spectra and signal to noise. Phys Med Biol. 2007;52:4047–61.
Mori I. CT ni okeru gashitsuno butsurishihyo ni tsuite (Physical image quality indexes of CT). Innervision. 2015;30:2–4.
Kohler T, Proska T. Noise properties of maximum likelihood reconstruction with edge-preserving regularization in transmission tomography. In: Fully 3D 2011 Proceedings, p. 263–266.
Ito M, Okubo T, Takahashi N. X-sen CT ni okeru MTF sokutei seido (Measurement accuracy of MTF for X-ray CT). Tohoku J Radiol Technol. 2006;15:146–7.
Acknowledgements
This work was supported by JSPS KAKENHI Grant number 15K09883. Parts of this work were presented at the 102nd Scientific Assembly of Radiological Society of North America in Chicago (2016), and at the Fourth Meeting of Japanese Society of CT Technology in Tokyo (2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This study was not performed on animal or human samples; therefore, it does not deal with animal and human ethical issues and informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Appendices
Appendix A: Registration of many images of wire to form a PSF
The 3D data \( D_{k} \left( {i,j} \right) \) are fitted using a model function \( g\left( {i,j,k} \right) \) that is a two-dimensional Gaussian for which the peak position gradually shifts with k:
In nature, \( i_{C} \left( k \right) \) and \( j_{C} \left( k \right) \) can be expressed without a square term because the wire is sufficiently straight. The reason for the square term is that it serves as an indicator of the accuracy of fitting. If the square term is not negligibly small, it means that the detected wire position may be inaccurate. This might occur rarely under extremely low-CNR conditions. In such a case, we have the option of using images with lower noise, such as those at a higher tube current, as surrogates for the fitting.
Using the wire center coordinates \( i_{C} \left( k \right) \) and \( j_{C} \left( k \right) \) determined as described above, the following \( D^{\prime}_{k} \left( {i,j} \right) \) is obtained as a coordinate-shifted version of \( D_{k} \left( {i,j} \right) \). Now, i and j are not indexes but real numbers. The registration is now completed because the wire center of \( D^{\prime}_{k} \left( {i,j} \right) \) is located exactly at \( i = j = 0 \) for all \( k \):
The primitive PSF is readily obtainable as the union of \( D^{\prime}_{k} \left( {i,j} \right) \). Here, \( \cup \) and \( \cup \) signify a union operation:
The explanation presented above describes the case of a single wire. However, plural wires of the same contrast are used for better performance at a low CNR. When m = 1 – M wires are used, each \( {\text{PSF}}_{0m} \left( {i,j} \right) \) is obtained for each wire in the manner presented above. Their union is \( {\text{PSF}}_{0} \left( {i,j} \right) \):
Appendix B: Correction of effects due to wire thickness and data processing
The frequency response correction function \( C\left( f \right) \) comprises three terms:
\( B\left( f \right) \) is the frequency response of the binning operation with bin size w or the Fourier transform of the rectangular function of width w. \( I\left( f \right) \) represents the frequency response of a linear interpolation operation for which the data pitch is w. Alternatively, it is the Fourier transform of a triangular function of which the base width is 2w. \( D\left( f \right) \) is the frequency response of the wire diameter d. Alternatively, it is the Fourier transform of a circular function with diameter d. \( J_{1} \) denotes the first-order Bessel function.
Appendix C: Base-level correction distance and truncation distance
The choices of the base-correction distance r and truncation distance t are important under low-CNR conditions. We explain our choices and rationale.
The choice of r is of primary importance. We also used the same value for t because no particular reason exists to use different values. We first observed PSFs under relatively high-CNR conditions. Visually, the PSF was well-diminished 2–3 mm from the center for all reconstruction kernels used for this study. Although small values for r and t are desirable for robustness against noise, we added some margin to avoid the subtle foot of the PSF. Therefore, we chose 3 mm for the Standard kernel and 4 mm for the others. The choice of 4 mm for the Lung kernel might require some explanation. Its PSF accompanies the ringing that continues beyond 4 mm. However, small positive/negative alternating values beyond r do not substantially affect the base-level appraisal. The truncation of the small ringing is also not of concern because it only limits the frequency resolution which is not required to depict the smooth MTF profile.
At a high CNR, the use of larger values for r and t is safer to maintain distance from the foot of PSF. However, at a low CNR, an overly large value is detrimental. Figure 13 shows how the choices of r and t affect the measurement at a low CNR. Among 20 repeated scans, the results of the first six scans are shown.
Effects of the base-correction distance r and truncation distance t for Al 0.3 at 50 mA. The upper row is for the FC13 kernel of Aquilion One (pCNR ≈ 5.9). The lower row is for the Standard kernel of Discovery CT750HD (pCNR ≈ 5.7). The left-side graphs show the area under the MTF curve. The right-graphs show the signal amount. The horizontal lines show the values obtained from high-pCNR ensemble-averaged images using r = t = 4 mm for the FC13 kernel and using r = t = 3 mm for the Standard kernel
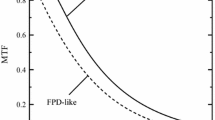
The trend of A (AUC of MTF) is closely correlated with that of S, as shown in the figure. When the values for r and t are too small, A is too high and S is too low. This is because the foot (positive value) of the PSF is incorrectly included in the data for the base-level appraisal. Consequently, the base-corrected PSF is shifted to lower values. S, the volume of PSF, is underestimated. This leads to an overestimate of MTF, because S is equal to the DC value of the Fourier transform of the PSF, and because the MTF is the Fourier transform of PSF divided by the DC value. On the other hand, a large value for r means a reduction in the area used for the base-level appraisal. When stochastic noise governs, the error of the base-level appraisal is inversely proportional to the square root of the area. A larger value of r will increase the stochastic error of the base-level appraisal. The base-corrected PSF is incorrectly shifted up or down by this stochastic error. When the PSF is shifted up, the measured S increases, and A decreases, as exemplified by the MTFs of the dashed line in Fig. 6. In short, overly small values for r and t cause a constant error, and overly large values cause a random error.
Figure 13 shows the extreme uncertainty in A and S when the values of r and t approach 7 mm. This is because our ROI size is only 26 × 26 pixels (= 16 × 16 mm2). Even at 3 or 4 mm, which we chose for our study, some variation in A and S is observable, which could be attributable to the stochastic error of the base-level appraisal. Even so, we chose a small ROI for this study for the efficiency of analyzing hundreds of cases using tens of thousands of images because the use of more pixels means greater computational demands.
About this article
Cite this article
Tominaga, C., Azumi, H., Goto, M. et al. Tilted-wire method for measuring resolution properties of CT images under extremely low-contrast and high-noise conditions. Radiol Phys Technol 11, 125–137 (2018). https://doi.org/10.1007/s12194-018-0443-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12194-018-0443-8