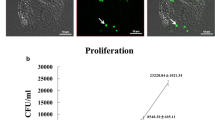
Abstract
Bovine viral diarrhea (BVD) is a worldwide infectious disease caused by bovine viral diarrhea virus (BVDV) infection, which invades the placenta, causes abortion, produces immune tolerance and continuously infects calves, and causes huge economic losses to the cattle industry. The endoplasmic reticulum (ER) is an important organelle in cells, which is prone to ER stress after being stimulated by pathogens, thus activating the ER stress–related apoptosis. Studies have confirmed that BVDV can utilize the ER of its host to complete its own proliferation and stimulate ER stress to a certain extent. However, the role of ER stress in BVDV infecting bovine placental trophoblast cells (BTCs) and inducing apoptosis is still unclear. We are using the cytopathic strain of BVDV (OregonC24Va), which can cause apoptosis of BTCs, as a model system to determine how ER stress induced by BVDV affects placental toxicity. We show that OregonC24Va can infect BTCs and proliferate in it. With the proliferation of BVDV in BTCs, ER stress–related apoptosis is triggered. The ER stress inhibitor 4-PBA was used to inhibit the ER stress of BTCs, which not only inhibited the proliferation of BVDV, but also reduced the apoptosis of BTCs. The ER stress activator Tg can activate ER stress–related apoptosis, but the proliferation of BVDV does not change in BTCs. Therefore, BVDV utilizes the UPR of activated ER stress to promote the proliferation of BVDV in the early stage of infection, and activates the ER stress–related apoptosis of BTCs in the later stage with the virus proliferation to promote the cell apoptosis and further spread of the virus. Our research provides a new theoretical basis for exploring the placental infection and vertical transmission of BVDV.







Similar content being viewed by others
Data availability
All data generated or analyzed in this study are available from the corresponding author on reasonable request.
Abbreviations
- ER :
-
Endoplasmic reticulum
- BVDV :
-
Bovine viral diarrhea virus
- BVD :
-
Bovine viral diarrhea
- BTCs :
-
Bovine placental trophoblast cells
- CHOP :
-
C/EBP homologous protein
- GRP78 :
-
Glucose-regulated protein, 78 KDa
- UPR :
-
Unfolded protein response
- IRE-1 :
-
Inositol-requiring enzyme1
- ATF6 :
-
Activating transcription factor 6
- PERK :
-
PKR-like ER kinase
- XBP1 :
-
X-box-binding protein
- ERAD :
-
Endoplasmic reticulum–associated degradation
- GADD153 :
-
Growth arrest and DNA-damage inducible gene153
- JNK :
-
C-Jun N-terminal kinase
- PI :
-
Persistently infected
- CP :
-
Cytopathogenic
- NCP :
-
Noncytopathic
- Bcl-2 :
-
B cell lymphoma-2
- ORF :
-
Open reading frame
- IRF-3 :
-
Interferon regulatory factor 3
- Tg :
-
Thapsigargin
- 4-PBA :
-
4-Phenylbutyric acid
- IFN :
-
Interferon
- MDBK :
-
Madin-Darby bovine kidney
- CPE :
-
Cytopathic effect
- WNV :
-
West Nile virus
- IBV :
-
Infectious bronchitis virus
- DEV :
-
Duck enteritis virus
- Bax :
-
BCL2-associated X protein
- Bak :
-
Bcl-2 homologous antagonist/killer
- PRV :
-
Pseudorabies virus
- MDV :
-
Marek’s disease virus
- ZIKV :
-
Zika virus
- DENV :
-
Dengue virus
- eIF2α :
-
Eukaryotic initiation factor 2α
References
Avila MF, Cabezas R, Torrente D, Gonzalez J, Morales L, Alvarez L, Capani F, Barreto GE (2013) Novel interactions of GRP78: UPR and estrogen responses in the brain. Cell Biol Int 37(6):521–532. https://doi.org/10.1002/cbin.10058
Bolin SR, McClurkin AW, Cutlip RC, Coria MF (1985) Severe clinical disease induced in cattle persistently infected with noncytopathic bovine viral diarrhea virus by superinfection with cytopathic bovine viral diarrhea virus. Am J Vet Res 46(3):573–576
Capellini I (2012) The evolutionary significance of placental interdigitation in mammalian reproduction: contributions from comparative studies. Placenta 33(10):763–768. https://doi.org/10.1016/j.placenta.2012.07.004
Cybulsky Andrey V (2017) Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol 13(11):681–696. https://doi.org/10.1038/nrneph.2017.129
Fenglei Chen, Qian Li, Zhe Zhang, Pengfei Lin, Lanjie Lei, Aihua Wang, Yaping Jin (2015) Endoplasmic reticulum stress cooperates in zearalenone-induced cell death of RAW 2647 Macrophages. Int J Mol Sci 16(8):19780–95. https://doi.org/10.3390/ijms160819780
Fulton RW (2015) Impact of species and subgenotypes of bovine viral diarrhea virus on control by vaccination. Animal Health Research Reviews 16(1):40–54. https://doi.org/10.1017/S1466252315000079
Fung TS, Liao Y, Liu DX (2014) The endoplasmic reticulum stress sensor IRE1α protects cells from apoptosis induced by the coronavirus infectious bronchitis virus. J Virol 88(21):12752–12764. https://doi.org/10.1128/JVI.02138-14
Jaskulska A, Janecka AE, Gach-Janczak K (2020) Thapsigargin-from traditional medicine to anticancer drug. Int J Mol Sci 22(1):4. https://doi.org/10.3390/ijms22010004
Jiang C, Yang Y, Huang C, Whitelaw B (2014) Promoter characterization and functional association with placenta of porcine MAGEL2. Gene 15 547(1):63–9. https://doi.org/10.1016/j.gene.2014.06.022
Hilbe M, Girao V, Bachofen C, Schweizer M, Zlinszky K, Ehrensperger F (2013) Apoptosis in bovine viral diarrhea virus (BVDV)-induced mucosal disease lesions: a histological, immunohistological, and virological investigation. Vet Pathol 50(1):46–55. https://doi.org/10.1177/0300985812447826
Hiroi T, Wei H, Hough C, Leeds P, Chuang DM (2005) Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2. Pharmacogenomics J 5(2):102–111. https://doi.org/10.1038/sj.tpj.6500296
Hue H (1999) Epidemiological features and economical importance of bovine virus diarrhoea virus (BVDV) infections. Vet Microbiol 64(2–3):89–107. https://doi.org/10.1016/s0378-1135(98)00262-4
Gao X, Niu C, Wang Z, Jia S, Han M, Ma Y, Guan X, Wang Li, Qiao X, Yigang Xu (2021) Comprehensive analysis of lncRNA expression profiles in cytopathic biotype BVDV-infected MDBK cells provides an insight into biological contexts of host-BVDV interactions. Virulence 12(1):20–34. https://doi.org/10.1080/21505594.2020.1857572
Karniychuk UU, Nauwynck HJ (2013) Pathogenesis and prevention of placental and transplacental porcine reproductive and respiratory syndrome virus infection. Vet Res 7 44(1):95. https://doi.org/10.1186/1297-9716-44-95
Kaur B, Bhat A, Chakraborty R, Adlakha K, Sengupta S, Roy S, Chakraborty K (2018) Proteomic profile of 4-PBA treated human neuronal cells during ER stress. Molecular Omics 14(1):53–63. https://doi.org/10.1039/c7mo00114b
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7(12):1013–1030. https://doi.org/10.1038/nrd2755
Kohlrausch FB, Keefe DL (2020) Telomere erosion as a placental clock: from placental pathologies to adverse pregnancy outcomes. Placenta 97:101–107. https://doi.org/10.1016/j.placenta.2020.06.022
Kolpikova EP, Tronco AR, Hartigh A, Jackson KJ, Iwawaki T, Fink SL (2020) IRE1α Promotes Zika virus infection via XBP1. Viruses 12(3):278. https://doi.org/10.3390/v12030278
Lee JK, Oh SJ, Park H, Shin OS (2019) Recent updates on research models and tools to study virus-host interactions at the placenta. Viruses 18 12(1):5. https://doi.org/10.3390/v12010005
Lamb Heather K (2006) Mee Christopher; Xu Weiming; Liu Lizhi; Blond Sylvie; Cooper Alan; Charles Ian G; Hawkins Alastair R (2006) The affinity of a major Ca2+ binding site on GRP78 is differentially enhanced by ADP and ATP. J Biol Chem 281(13):8796–8805. https://doi.org/10.1074/jbc.M503964200
Li S, Kong L, Yu X (2015) The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit Rev Microbiol 41(2):150–164. https://doi.org/10.3109/1040841X.2013.813899
Maude B, Sandra F, Peter T (2010) Multiple pathways involved in porcine parvovirus cellular entry and trafficking toward the nucleus. J Virol 84:7782–7792. https://doi.org/10.1128/JVI.00479-10
Medigeshi GR, Lancaster AM, Hirsch AJ, Thomas Briese W, Lipkin I, Defilippis V, Früh K, Mason PW, Nikolich-Zugich J, Nelson JA (2007) West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis. J Virol 81(20):10849–60. https://doi.org/10.1128/JVI.01151-07
Morère L et al (2015) Ex vivo model of congenital cytomegalovirus infection and new combination therapies. Placenta 36(1):41–47. https://doi.org/10.1016/j.placenta.2014.11.003
Nair VP, Anang S, Subramani C, Madhvi A, BakshiSrivastava KA, Shalimar N, Ranjith B, Kumar CT, Surjit M (2016) Endoplasmic reticulum stress induced synthesis of a novel viral factor mediates efficient replication of genotype-1 hepatitis e virus. PLoS Pathog 12(4):e1005521. https://doi.org/10.1371/journal.ppat.1005521
Neerukonda SN, Katneni UK, Bott M, Golovan SP, Parcells MS (2018) Induction of the unfolded protein response (UPR) during Marek’s disease virus (MDV) infection. Virology 522:1–12. https://doi.org/10.1016/j.virol.2018.06.016
Parry S, Holder J, Strauss JF (1997) Mechanisms of trophoblast-virus interaction. J Reprod Immunol 15 37(1):25–34. https://doi.org/10.1016/s0165-0378(97)00071-5
Reid DW, Campos RK, Child JR, Zheng T, Chan K, Bradrick SS, Vasudevan SG, Garcia-Blanco MA, Nicchitta CV (2018) Dengue virus selectively annexes endoplasmic reticulum-associated translation machinery as a strategy for co-opting host cell protein synthesis. J Virol 92(7):e01766-e1817. https://doi.org/10.1128/JVI.01766-17
Rosenfeld Cheryl S (2020) The placenta-brain-axis. J Neurosci Res 99(1):271–283. https://doi.org/10.1002/jnr.24603
Salcedo SP, Chevrier N, Lacerda TLS, Amara AB, Gerart S, Gorvel VA, de Chastellier C, Blasco JM, Mege J-L, Gorvel J-P (2013) Pathogenic brucellae replicate in human trophoblasts. J Infect Dis 207(7):1075–83. https://doi.org/10.1093/infdis/jit007
Samali A, Fitzgerald U, Deegan S, Gupta S (2010) Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int J Cell Biol 2010(2010):830307. https://doi.org/10.1155/2010/830307
Vassilev VB, Donis RO (2000) Bovine viral diarrhea virus induced apoptosis correlates with increased intracellular viral RNA accumulation. Virus Res 25 69(2):95–107. https://doi.org/10.1016/S0168-1702(00)00176-3
Wang S, Hou P, Pan W, He W, He DC, Wang H, He H (2021) DDIT3 targets innate immunity via the DDIT3-OTUD1-MAVS pathway to promote bovine viral diarrhea virus replication. J Virol 95(6):e02351-e2420. https://doi.org/10.1128/JVI.02351-20
Yamauchi Y, Greber UF (2016) Principles of virus uncoating: cues and the snooker ball. Traffic 17(6):569–592. https://doi.org/10.1111/tra.12387
Yang S, Zhu J, Zhou X, Wang H, Li X, Zhao A (2019) Induction of the unfolded protein response (UPR) during pseudorabies virus infection. Vet Microbiol 239:108485. https://doi.org/10.1016/j.vetmic.2019.108485
Yangzi Z, Wang Xuan H, Yutong AW, Piao Z, Xia Y, Zhengchang W, Ming W (2020) Duck enteritis virus infection suppresses viability and induces apoptosis and endoplasmic reticulum stress in duck embryo fibroblast cells via the regulation of Ca 2. J Vet Med Sci 3 83(3):549–557. https://doi.org/10.1292/jvms.19-0584
Yeşilbağ K, Alpay G, Becher P (2017) Variability and global distribution of subgenotypes of bovine viral diarrhea virus. Viruses 9(6):E128. https://doi.org/10.3390/v9060128
Yin H, Zhao L, Jiang X, Li S, Huo H, Chen H (2017) DEV induce autophagy via the endoplasmic reticulum stress related unfolded protein response. PLoS ONE 12(12):e0189704. https://doi.org/10.1371/journal.pone.0189704
Yitagesu E, Jackson W, Kebede N, Smith W, Fentie T (2021) Prevalence of bovine abortion, calf mortality, and bovine viral diarrhea virus (BVDV) persistently infected calves among pastoral, peri-urban, and mixed-crop livestock farms in central and Northwest Ethiopia. BMC Vet Res 19 17(1):87. https://doi.org/10.1186/s12917-021-02798-w
Zhang K, Kaufman RJ (2008) From endoplasmic-reticulum stress to the inflammatory response. Nature 454:455–462. https://doi.org/10.1038/nature07203
Zhu H, Zhou H (2021) Novel Insight into the role of endoplasmic reticulum stress in the pathogenesis of myocardial ischemia-reperfusion injury. Oxid Med Cell Longev 2021:5529810. https://doi.org/10.1155/2021/5529810
Zhu P, Hu S, Jin Q, Li D, Tian F, Toan S, Li Y, Zhou H, Chen Y (2018) Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: a mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol 16:157–168. https://doi.org/10.1016/j.redox.2018.02.019
Acknowledgements
This research was supported by the National Natural Science Foundation of China (NO. 31802263, 31802258) and the Zhejiang Provincial Natural Science Foundation (NO. LY22C18000).
Author information
Authors and Affiliations
Contributions
NHY, QJS, and XGW performed the experiments and collected and interpreted the data. XHS, XLQ, and KX provided initial help with the analysis. ZHZ, ZW, and YJ collected the samples. XGW and NHY conceptualized and wrote the manuscript. LFX reviewed the manuscript prior to publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experimental and surgical procedures involving animals were approved by the Beijing Laboratory Animal Management Committee.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, N., Song, Q., Jin, Y. et al. Replication of standard bovine viral diarrhea strain OregonC24Va induces endoplasmic reticulum stress–mediated apoptosis of bovine trophoblast cells. Cell Stress and Chaperones 28, 49–60 (2023). https://doi.org/10.1007/s12192-022-01300-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-022-01300-1