Abstract
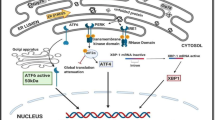
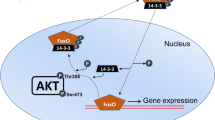
The unfolded protein response (UPR) is a wide-ranging cellular response to accumulation of malfolded proteins in the endoplasmic reticulum (ER) and acts as a quality control mechanism to halt protein processing and repair/destroy malfolded proteins under stress conditions of many kinds. Among vertebrate species, amphibians experience the greatest challenges in maintaining water and osmotic balance, the high permeability of their skin making them very susceptible to dehydration and challenging their ability to maintain cellular homeostasis. The present study evaluates the involvement of the UPR in dealing with dehydration-mediated disruption of protein processing in the tissues of African clawed frogs, Xenopus laevis. This primarily aquatic frog must deal with seasonal drought conditions in its native southern Africa environment. Key markers of cellular stress that impact protein processing were identified in six tissues of frogs that had lost 28% of total body water, as compared with fully hydrated controls. This included upregulation of glucose-regulated proteins (GRPs) that are resident chaperones in the ER, particularly 2–ninefold increases in GRP58, GRP75, and/or GRP94 in the lung and skin. Activating transcription factors (ATF3, ATF4, ATF6) that mediate UPR responses also responded to dehydration stress, particularly in skeletal muscle where both ATF3 and ATF4 rose strongly in the nucleus. Other protein markers of the UPR including GADD34, GADD153, EDEM, and XBP-1 also showed selective upregulation in frog tissues in response to dehydration and nuclear levels of the transcription factors XBP-1 and P-CREB rose indicating up-regulation of genes under their control.




Similar content being viewed by others
Abbreviations
- ATF:
-
Activating transcription factor
- CREB:
-
cAMP response element-binding protein
- EDEM:
-
ER degradation-enhancing alpha-mannosidase-like 1
- eIF2α:
-
Eukaryotic initiation factor 2α
- ER:
-
Endoplasmic reticulum
- ERAD:
-
ER-associated degradation
- ERK:
-
Extracellular signal-regulated kinase
- FoxO:
-
Forkhead box transcription factor, subgroup O
- GAAD:
-
Growth arrest and DNA damage protein
- GRP:
-
Glucose-regulated protein
- HSP:
-
Heat shock protein
- IRE1:
-
Inositol-requiring kinase 1
- PDI:
-
Protein disulfide isomerase
- PERK:
-
PKR-like eukaryotic initiation factor 2α kinase
- SDS-PAGE:
-
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis
- UPR:
-
Unfolded protein response
- XBP1:
-
X-box binding protein 1
References
Ashcroft F (2000) Life at the extremes. HarperCollins Publishers, London, pp 134–138
Balinsky JB, Choritz EL, Coe CGL, van der Schans GS (1967) Amino acid metabolism and urea synthesis in naturally aestivating Xenopus laevis. Comp Biochem Physiol 22:59–68. https://doi.org/10.1016/0010-406x(67)90166-1
Biggar KK, Biggar Y, Storey KB (2015) Identification of a novel dehydration responsive gene, drp10, from the African clawed frog, Xenopus laevis. J Exp Zool A 323:375–381. https://doi.org/10.1002/jez.1930
Brush MH, Weiser DC, Shenolikar S (2003) Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol 23:1292–1303. https://doi.org/10.1128/MCB.23.4.1292-1303.2003
Cartledge VA, Withers PC, McMaster KA, Thompson GG, Bradshaw SD (2006) Water balance of field-excavated aestivating Australian desert frogs, the cocoon forming Neobatrachus aquilonius and the non-cocooning Notaden nichollsi (Amphibia: Myobatrachidae). J Exp Biol 209:3309–3321. https://doi.org/10.1371/journal.pone.0086661
Carvalho HH, Brustolini OJB, Pimenta MR, Mendes GC, Gouveia BC, Silva PA, Silva JC, Mota CS, Soares-Ramos JRL, Fontes EPB (2014) The molecular chaperone binding protein BiP prevents leaf dehydration-induced cellular homeostasis disruption. PLoS ONE 9:e86661. https://doi.org/10.1371/journal.pone.0086661
Chakrabarti A, Chen AW, Varner JD (2011) A review of the mammalian unfolded protein response. Biotechnol Bioeng 108:2777–2793. https://doi.org/10.1002/bit.23282
do Amaral MCF, Frisbie J, Crum RJ, Goldstein DL, Krane CM (2020) Hepatic transcriptome of the freeze-tolerant Cope’s gray treefrog, Dryophytes chrysoscelis: responses to cold acclimation and freezing. BMC Genomics 21:226. https://doi.org/10.1186/s12864-020-6602-4
Dores-Silva PR, Barbosa LRS, Ramos CHI, Borges JC (2015) Human mitochondrial Hsp70 (mortalin): shedding light on ATPase activity, interaction with adenosine nucleotides, solution structure and domain organization. PLoS ONE 10(1):e0117170
Dragosits M, Stadlmann J, Graf A, Gasser B, Maurer M, Sauer M, Krei DP, Altmann F, Mattanovich D (2010) The response to unfolded protein is involved in osmotolerance of Pichia pastoris. BMC Genomics 11:207. https://doi.org/10.1186/1471-2164-11-207
Dubaissi E, Rousseau K, Hughes GW, Ridley C, Grencis RK, Roberts IS, Thornton DJ (2018) Functional characterization of the mucus barrier on the Xenopus tropicalis skin surface. Proc Natl Acad Sci USA 115:726–773. https://doi.org/10.1073/pnas.1713539115
Flachbartová Z, Kovacech B (2013) Mortalin - a multipotent chaperone regulating cellular processes ranging from viral infection to neurodegeneration. Acta Virol 57:3–15. https://doi.org/10.4149/av_2013_01_3
Fusakio ME, Willy JA, Wang Y, Mirek ET, Al Baghdadi RJ, Adams CM, Anthony TG, Wek RC (2016) Transcription factor ATF4 directs basal and stress-induced gene expression in the unfolded protein response and cholesterol metabolism in the liver. Mol Biol Cell 27:1536–1551. https://doi.org/10.1091/mbc.E16-01-0039
Gething MJ (1999) Role and regulation of the ER chaperone BiP. Cell Devel Biol 10:465–472. https://doi.org/10.1006/scdb.1999.0318
Giraud-Billoud M, Rivera-Ingraham GA, Moreira DC, Burmester T, Castro-Vazquez A, Carvajalino-Fernández JM, Dafre A, Niu C, Tremblay N, Paital B, Rosa R, Storey JM, Vega IA, Zhang W, Yepiz-Plascencia G, Zenteno-Savin T, Storey KB, Hermes-Lima M (2019) Twenty years of the “preparation for oxidative stress” (POS) theory: ecophysiological advantages and molecular strategies. Comp Biochem Physiol A 234:36–49. https://doi.org/10.1016/j.cbpa.2019.04.004
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633. https://doi.org/10.1006/scdb.1999.0318
Hillman SS (1978) Some effects of dehydration on internal distribution of water and solutes in Xenopus laevis. Comp Biochem Physiol 61:303–307. https://doi.org/10.1016/0300-9629(78)90113-5
Hillman SS (1978) The roles of oxygen delivery and electrolyte levels in the dehydrational death of Xenopus laevis. J Comp Physiol 128:169–175. https://doi.org/10.1007/BF00689481
Hillman SS (2018) Anuran amphibians as comparative models for understanding extreme dehydration tolerance: a unique negative feedback lymphatic mechanism for blood volume regulation. Am J Physiol Regul Integr Comp Physiol 315:R790–R798. https://doi.org/10.1152/ajpregu.00160.2018
Hoffman SM, Chapman DG, Lahue KG, Cahoon JM, Rattu GK, Daphtary N, Aliyeva M, Fortner KA, Erzurum SC, Comhair SAA, Woodruff PG, Bhakta N, Dixon AE, Irvin CG, Janssen-Heininger YMW, Poynter ME, Anathy V (2015) Protein disulfide isomerase–endoplasmic reticulum resident protein 57 regulates allergen-induced airways inflammation, fibrosis, and hyperresponsiveness. J Allergy Clin Immunol 137:822–832. https://doi.org/10.1016/j.jaci.2015.08.018
Jones RM (1980) Metabolic consequences of accelerated urea synthesis during seasonal dormancy of spadefoot toads, Scaphiopus couchii and Scaphiopus multiplicatus. J Exp Biol 212:255–267. https://doi.org/10.1002/jez.1402120212
Kikuchi D, Tanimoto K, Nakayama K (2016) CREB is activated by ER stress and modulates the unfolded protein response by regulating the expression of IRE1α and PERK. Biochim Biophys Res Commun 469:243–250. https://doi.org/10.1016/j.bbrc.2015.11.113
Kozutsumi Y, Segal M, Normington K, Gething M-J, Sambrook J (1988) The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332(6163):462–464. https://doi.org/10.1038/332462a0
Krivoruchko A, Storey KB (2013) Activation of the unfolded protein response during anoxia exposure in the turtle Trachemys scripta elegans. Mol Cell Biochem 374:91–103. https://doi.org/10.1007/s11010-012-1508-3
Lee AS (2001) The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci 26:504–510. https://doi.org/10.1016/s0968-0004(01)01908-9
Lee KPK, Dey M, Neculai D, Cao C, Dever TE, Sicheri F (2008) Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in non-conventional RNA splicing. Cell 132:89–100. https://doi.org/10.1016/j.cell.2007.10.057
Loveridge JP (1976) Strategies of water conservation in Southern African frogs. African Zool 11:319–333. https://doi.org/10.1080/00445096.1976.11447538
Luo S, Baumeister P, Yang S, Abcouwer SF, Lee AS (2003) Induction of Grp78/BiP by translational block. J Biol Chem 278:37375–37385. https://doi.org/10.1074/jbc.M303619200
Luu BE, Wijenayake S, Malik AI, Storey KB (2018) The regulation of heat shock proteins in response to dehydration in Xenopus laevis. Cell Stress Chaperones 23:45–53. https://doi.org/10.1007/s12192-017-0822-9
Malik AI, Storey KB (2009) Activation of antioxidant defense during dehydration stress in the African clawed frog. Gene 442:99–107. https://doi.org/10.1016/j.gene.2009.04.007
Malik AI, Storey KB (2009) Activation of extracellular signal-regulated kinases during dehydration in the African clawed frog, Xenopus laevis. J Exp Biol 212:2595–2603. https://doi.org/10.1242/jeb.030627
Malik AI, Storey KB (2011) Transcriptional regulation of antioxidant enzymes by FoxO1 under dehydration stress. Gene 485:114–119. https://doi.org/10.1016/j.gene.2011.06.014
Mamady H, Storey KB (2006) Up-regulation of the endoplasmic reticulum molecular chaperone GRP78 during hibernation in thirteen-lined ground squirrels. Mol Cell Biochem 292:89–98. https://doi.org/10.1007/s11010-006-9221-8
McQuiston A, Diehl JA (2017) Recent insights into PERK-dependent signaling from the stressed endoplasmic reticulum. FResearch 6:1897. https://doi.org/10.12688/f1000research.12138.1
Molinari M, Calanca V, Galli C, Lucca P, Paganetti P (2003) Role of EDEM in the release of misfolded glycoproteins from the calnexin cycle. Science 299:1397–1400. https://doi.org/10.1126/science.1079474
Muir TJ, Costanzo JP, Lee RE Jr (2007) Osmotic and metabolic responses to dehydration and urea-loading in a dormant, terrestrially hibernating frog. J Comp Physiol B 177:917–926. https://doi.org/10.1007/s00360-007-0190-3
Ni M, Lee AS (2007) ER chaperones in mammalian development and human diseases. FEBS Lett 581:3641–3651. https://doi.org/10.1016/j.febslet.2007.04.045
Park C-J, Park JM (2019) Endoplasmic reticulum plays a critical role in integrating signals generated by both biotic and abiotic stress in plants. Front Plant Sci 10:399. https://doi.org/10.3389/fpls.2019.00399
Schroder M (2008) Endoplasmic reticulum stress responses. Cell Mol Life Sci 65:862–894. https://doi.org/10.1007/s00018-007-7383-5
Schroder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789
Shishkin SS, Eremina LS, Kovalev LI (2013) AGR2, ERp57/GRP58, and some other human protein disulfide isomerases. Biochem Mosc 78:1415–1430. https://doi.org/10.1134/S000629791313004X
Storey KB (2002) Life in the slow lane: molecular mechanisms of estivation. Comp Biochem Physiol A 133:733–754. https://doi.org/10.1016/s1095-6433(02)00206-4
Storey KB, Storey JM (2004) Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev Camb Philos Soc 79:207–233. https://doi.org/10.1017/s1464793103006195
Storey KB, Storey JM (2007) Putting life on ‘pause’ – molecular regulation of hypometabolism. J Exp Biol 210:1700–1714. https://doi.org/10.1242/jeb.02716
Storey KB, Storey JM (2017) Molecular physiology of freeze tolerance in vertebrates. Physiol Rev 97:623–665. https://doi.org/10.1152/physrev.00016.2016
Storey JM, Storey KB (2019) In defense of proteins: chaperones respond to freezing, anoxia or dehydration stress in tissues of freeze tolerant wood frogs. J Exp Zool 331:392–402. https://doi.org/10.1002/jez.2306
Tinsley RC, Kobel HR (1996) The Biology of Xenopus. Oxford University Press, Oxford
Turano C, Gaucci U, Grillo C, Chichiarelli S (2011) ERp57/GRP58: a protein with multiple functions. Cell Mol Biol Let 16:539–563. https://doi.org/10.2478/s11658-011-0022-z
Varga JFA, Bui-Marinos MP, Katzenback BA (2019) Frog skin innate immune defences: sensing and surviving pathogens. Front Immunol 9:3128. https://doi.org/10.3389/fimmu.2018.03128
Wang X, Zhang T, Mao H, Mi Y, Zhong B, Wei L, Liu X, Hu C (2016) Grass carp (Ctenopharyngodon idella) ATF6 (activating transcription factor 6) modulates the transcriptional level of GRP78 and GRP94 in CIK cells. Fish Shellfish Immunol 52:65–73. https://doi.org/10.1016/j.fsi.2016.03.028
Wright P, Anderson P, Weng L, Frick N, Wong WP, Ip YK (2004) The crab-eating frog, Rana cancrivora, up-regulates hepatic carbamoyl phosphate synthetase I activity and tissue osmolyte levels in response to increased salinity. J Exp Zool A 301:559–568. https://doi.org/10.1002/jez.a.54
Wu C-W, Biggar KK, Storey KB (2013) Dehydration mediated microRNA response in the African clawed frog Xenopus laevis. Gene 529:269–275. https://doi.org/10.1016/j.gene.2013.07.064
Wu C-W, Tessier SN, Storey KB (2017) Regulation of the insulin-Akt signaling pathway and glycolysis during dehydration stress in the African clawed frog Xenopus laevis. Biochem Cell Biol 95:663–671. https://doi.org/10.1139/bcb-2017-0117
Yancey P (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830. https://doi.org/10.1242/jeb.01730
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891. https://doi.org/10.1016/s0092-8674(01)00611-0
Zhang Y, Luu BE, Storey KB (2018) FoxO4 activity is regulated by phosphorylation and the cellular environment during dehydration in the African clawed frog, Xenopus laevis. Biochim Biophys 1862:1721–1728. https://doi.org/10.1016/j.bbagen.2018.05.002
Zhu G, Lee AS (2015) Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol 230:1413–1420. https://doi.org/10.1002/jcp.24923
Acknowledgements
This research was supported by a Discovery grant from the Natural Sciences and Engineering Research Council of Canada to KBS (#6793); KBS holds the Canada Research Chair in Molecular Physiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malik, A.I., Storey, J.M. & Storey, K.B. Regulation of the unfolded protein response during dehydration stress in African clawed frogs, Xenopus laevis. Cell Stress and Chaperones 28, 529–540 (2023). https://doi.org/10.1007/s12192-022-01275-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-022-01275-z