Abstract
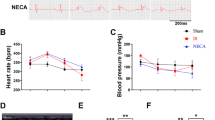
Type 2 diabetic hearts are more vulnerable to myocardial ischemia reperfusion (MIR) injury, which involves decreased mitophagy status with unknown mechanisms. MitoQ, a mitochondria-targeted antioxidant, has been shown to have protection against ischemia reperfusion injury through upregulating mitophagy. The aim of this study was to investigate the effects of MitoQ on myocardium during MIR injury in type 2 diabetes (T2D). Herein, this study discovered that type 2 diabetic hearts with PINK1/Parkin downregulation suffered more MIR injury accompanied by reduced mitophagy. Treatment with MitoQ significantly decreased the levels of CK-MB, LDH, myocardial infarction, myocardial pathological damage, and cardiomyocytes apoptosis, while it improved cardiac function, mitophagy status, and PINK1/Parkin pathway in vivo study. Furthermore, MitoQ significantly reduced high glucose/high fat and hypoxia/reoxygenation induced injury in H9C2 cells as evidenced by reduced cardiomyocytes apoptosis and ROS production, and increased cell viability, the level of mitochondrial membrane potential, PINK1/Parkin expression. However, mitochondrial division inhibitor (mdivi-1), an inhibitor of mitophagy, reversed the improvement and protein expression levels of PINK1/Parkin pathway in vitro models. In conclusion, MIR induced more severe damage in T2D by reduction of mitophagy. MitoQ can confer cardioprotection following MIR in T2D by mitophagy up-regulation via PINK1/Parkin pathway.






Similar content being viewed by others
Data availability
All the data can be obtained from the corresponding authors.
Abbreviations
- CCK-8:
-
Cell counting kit-8
- CK-MB:
-
Creatine kinase-MB
- DCFH-DA:
-
Dichloro-dihydro-fluorescein diacetate
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBG:
-
Fasting blood glucose
- FBS:
-
Fetal bovine serum
- HG:
-
High glucose
- HR:
-
Hypoxia/reoxygenation
- HFD:
-
High-fat diet
- HR:
-
Heart rate
- LAD:
-
Left anterior descending coronary artery
- LDH:
-
Lactate dehydrogenase
- LVSP:
-
Left ventricular systolic blood pressure
- + dp/dtmax:
-
LVSP maximum increase rates
- − dp/dtmax:
-
LVSP maximum decrease rates
- MIR:
-
Myocardial ischemia reperfusion
- TEM:
-
Transmission electron microscopy
- T2D:
-
Type 2 diabetes
- 7-AAD:
-
7-Amino-actinomycin
References
Bordt EA, Clerc P, Roelofs BA et al (2017) The putative Drp1 inhibitor mdivi-1 is a reversible mitochondrial complex I inhibitor that modulates reactive oxygen species. Dev Cell 40(6):583–594.e586https://doi.org/10.1016/j.devcel.2017.02.020
Bragg F, Holmes MV, Iona A et al (2017) Association between diabetes and cause-specific mortality in rural and urban areas of China. Jama 317(3):280–289. https://doi.org/10.1001/jama.2016.19720
Bueno M, Lai YC, Romero Y et al (2015) PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 125(2):521–538. https://doi.org/10.1172/jci74942
Durga Devi T, Babu M, Mäkinen P et al (2017) Aggravated postinfarct heart failure in type 2 diabetes is associated with impaired mitophagy and exaggerated inflammasome activation. Am J Pathol 187(12):2659–2673. https://doi.org/10.1016/j.ajpath.2017.08.023
Emanuele S, Lauricella M, D'Anneo A et al (2020) p62: friend or foe? Evidences for oncojanus and neurojanus roles. Int J Mol Sci 21(14). https://doi.org/10.3390/ijms21145029
Escribano-Lopez I, Diaz-Morales N, Rovira-Llopis S et al (2016) The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol 10:200–205. https://doi.org/10.1016/j.redox.2016.10.017
Feng Y, Madungwe NB, da Cruz Junho CV et al (2017) Activation of G protein-coupled oestrogen receptor 1 at the onset of reperfusion protects the myocardium against ischemia/reperfusion injury by reducing mitochondrial dysfunction and mitophagy. Br J Pharmacol 174(23):4329–4344. https://doi.org/10.1111/bph.14033
Guan L, Che Z, Meng X et al (2019) MCU Up-regulation contributes to myocardial ischemia-reperfusion Injury through calpain/OPA-1-mediated mitochondrial fusion/mitophagy Inhibition. J Cell Mol Med 23(11):7830–7843. https://doi.org/10.1111/jcmm.14662
Hansson MJ, Llwyd O, Morin D et al (2015) Differences in the profile of protection afforded by TRO40303 and mild hypothermia in models of cardiac ischemia/reperfusion injury. Eur J Pharmacol 760:7–19. https://doi.org/10.1016/j.ejphar.2015.04.009
He Y, Zhou L, Fan Z et al (2018) Palmitic acid, but not high-glucose, induced myocardial apoptosis is alleviated by N-acetylcysteine due to attenuated mitochondrial-derived ROS accumulation-induced endoplasmic reticulum stress. Cell Death Dis 9(5):568. https://doi.org/10.1038/s41419-018-0593-y
Jin Q, Li R, Hu N et al (2018) DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol 14:576–587. https://doi.org/10.1016/j.redox.2017.11.004
Kageyama Y, Hoshijima M, Seo K et al(2014) Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. Embo J 33(23):2798–2813. https://doi.org/10.15252/embj.201488658
Kelso GF, Porteous CM, Coulter CV et al(2001) Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276(7):4588–4596. https://doi.org/10.1074/jbc.M009093200
Leng Y, Wu Y, Lei S et al (2018) Inhibition of HDAC6 Activity Alleviates myocardial ischemia/reperfusion injury in diabetic rats: potential role of peroxiredoxin 1 acetylation and redox regulation. Oxid Med Cell Longev 2018:9494052. https://doi.org/10.1155/2018/9494052
Li Q, Gao S, Kang Z et al (2018) Rapamycin enhances mitophagy and attenuates apoptosis after spinal ischemia-reperfusion injury. Front Neurosci 12:865. https://doi.org/10.3389/fnins.2018.00865
Li XX, Ling SK, Hu MY et al (2019) Protective effects of acarbose against vascular endothelial dysfunction through inhibiting Nox4/NLRP3 inflammasome pathway in diabetic rats. Free Radic Biol Med 145:175–186. https://doi.org/10.1016/j.freeradbiomed.2019.09.015
Liu X, Murphy MP, Xing W et al (2018) Mitochondria-targeted antioxidant MitoQ reduced renal damage caused by ischemia-reperfusion injury in rodent kidneys: Longitudinal observations of T2 -weighted imaging and dynamic contrast-enhanced MRI. Magn Reson Med 79(3):1559–1567. https://doi.org/10.1002/mrm.26772
Lyamzaev KG, Tokarchuk AV, Panteleeva AA et al (2018) Induction of autophagy by depolarization of mitochondria. Autophagy 14(5):921–924. https://doi.org/10.1080/15548627.2018.1436937
Mackenzie RM, Salt IP, Miller WH et al (2013) Mitochondrial reactive oxygen species enhance AMP-activated protein kinase activation in the endothelium of patients with coronary artery disease and diabetes. Clin Sci (Lond) 124(6):403–411. https://doi.org/10.1042/cs20120239
Morales PE, Arias-Duran C, Avalos-Guajardo Y et al (2019) Emerging role of mitophagy in cardiovascular physiology and pathology. Mol Aspects Med 100822. https://doi.org/10.1016/j.mam.2019.09.006
Mu J, Zhang D, Tian Y et al (2020) BRD4 inhibition by JQ1 prevents high-fat diet-induced diabetic cardiomyopathy by activating PINK1/Parkin-mediated mitophagy in vivo. J Mol Cell Cardiol 149:1–14. https://doi.org/10.1016/j.yjmcc.2020.09.003
Mukhopadhyay P, Horvath B, Zsengeller Z et al (2012) Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia-reperfusion: therapeutic potential of mitochondrially targeted antioxidants. Free Radic Biol Med 53(5):1123–1138. https://doi.org/10.1016/j.freeradbiomed.2012.05.036
Packer M (2018) Heart failure: the most important, preventable, and treatable cardiovascular complication of type 2 diabetes. Diabetes Care 41(1):11–13. https://doi.org/10.2337/dci17-0052
Paradies G, Paradies V, Ruggiero FM et al (2018) Mitochondrial bioenergetics and cardiolipin alterations in myocardial ischemia-reperfusion injury: implications for pharmacological cardioprotection. Am J Physiol Heart Circ Physiol 315(5):H1341–h1352. https://doi.org/10.1152/ajpheart.00028.2018
Qiu Z, Lei S, Zhao B et al (2017) NLRP3 inflammasome activation-mediated pyroptosis aggravates myocardial ischemia/reperfusion injury in diabetic rats. Oxid Med Cell Longev 2017:9743280. https://doi.org/10.1155/2017/9743280
Qiu Z, Ming H, Lei S et al (2021) Roles of HDAC3-orchestrated circadian clock gene oscillations in diabetic rats following myocardial ischaemia/reperfusion injury. Cell Death Dis 12(1):43. https://doi.org/10.1038/s41419-020-03295-y
Qiu Z, Wei Y, Song Q et al (2019) The role of myocardial mitochondrial quality control in heart failure. Front Pharmacol 10:1404. https://doi.org/10.3389/fphar.2019.01404
Rodriguez-Cuenca S, Cocheme HM, Logan A et al (2010) Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med 48(1):161–172. https://doi.org/10.1016/j.freeradbiomed.2009.10.039
Rossman MJ, Santos-Parker JR, Steward CAC et al (2018) Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 71(6):1056–1063. https://doi.org/10.1161/hypertensionaha.117.10787
Saeedi P, Petersohn I, Salpea P et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 157:107843. https://doi.org/10.1016/j.diabres.2019.107843
Shefa U, Jeong NY, Song IO et al (2019) Mitophagy links oxidative stress conditions and neurodegenerative diseases. Neural Regen Res 14(5):749–756. https://doi.org/10.4103/1673-5374.249218
Skulachev VP (2005) How to clean the dirtiest place in the cell: cationic antioxidants as intramitochondrial ROS scavengers. IUBMB Life 57(4–5):305–310. https://doi.org/10.1080/15216540500092161
Tong M, Saito T, Zhai P et al (2019) Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ Res 124(9):1360–1371. https://doi.org/10.1161/circresaha.118.314607
Turkieh A, El Masri Y, Pinet et al (2022) Mitophagy regulation following myocardial infarction. Cells 11(2). https://doi.org/10.3390/cells11020199
Wendt L, Vider J, Hoe LES et al (2020) Complex effects of putative DRP-1 inhibitors on stress responses in mouse heart and rat cardiomyoblasts. J Pharmacol Exp Ther 372(1):95–106. https://doi.org/10.1124/jpet.119.258897
Wu Y, Leng Y, Meng Q et al (2017) Suppression of excessive histone deacetylases activity in diabetic hearts attenuates myocardial ischemia/reperfusion injury via mitochondria apoptosis pathway. J Diabetes Res 2017:8208065. https://doi.org/10.1155/2017/8208065
Xiao L, Xu X, Zhang F et al (2017) The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol 11:297–311. https://doi.org/10.1016/j.redox.2016.12.022
Xu Y, Shen J, Ran Z (2020) Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy 16(1):3–17. https://doi.org/10.1080/15548627.2019.1603547
Yang J, Suo H, Song J (2021a) Protective role of mitoquinone against impaired mitochondrial homeostasis in metabolic syndrome. Crit Rev Food Sci Nutr 61(22):3857–3875. https://doi.org/10.1080/10408398.2020.1809344
Yang M, Linn BS, Zhang Y et al (2019) Mitophagy and mitochondrial integrity in cardiac ischemia-reperfusion injury. Biochim Biophys Acta Mol Basis Dis 1865(9):2293–2302. https://doi.org/10.1016/j.bbadis.2019.05.007
Yang M, Wang S, Fu S et al (2021b) Deletion of the E3 ubiquitin ligase, Parkin, exacerbates chronic alcohol intake-induced cardiomyopathy through an Ambra1-dependent mechanism. Br J Pharmacol 178(4):964–982. https://doi.org/10.1111/bph.15340
Yang X, Pan W, Xu G et al (2020) Mitophagy: A crucial modulator in the pathogenesis of chronic diseases. Clin Chim Acta 502:245–254. https://doi.org/10.1016/j.cca.2019.11.008
Yu LM, Dong X, Xue XD et al (2021) Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: Role of SIRT6. J Pineal Res 70(1):e12698. https://doi.org/10.1111/jpi.12698
Zeng Z, Yuan Q, Yu R et al (2019) Ameliorative effects of probiotic Lactobacillus paracasei NL41 on insulin sensitivity, oxidative stress, and beta-cell function in a type 2 diabetes mellitus rat model. Mol Nutr Food Res 63(22):e1900457. https://doi.org/10.1002/mnfr.201900457
Zhang W, Ren H, Xu C et al (2016) Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. Elife 5. https://doi.org/10.7554/eLife.21407
Zhang Y, Wang S, Chen X et al (2022) Liraglutide prevents high glucose induced HUVECs dysfunction via inhibition of PINK1/Parkin-dependent mitophagy. Mol Cell Endocrinol 545:111560. https://doi.org/10.1016/j.mce.2022.111560
Zhang Y, Wang Y, Xu J et al (2019) Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J Pineal Res 66(2):e12542. https://doi.org/10.1111/jpi.12542
Zhou H, Zhang Y, Hu S et al (2017) Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res 63(1). https://doi.org/10.1111/jpi.12413
Acknowledgements
The authors would like to thank the central laboratory at Renmin Hospital of Wuhan University (Wuhan, Hubei, China) for their support of our study.
Funding
This study was supported by grants from the National Natural Science Foundation of China (NSFC 81970722, 81671891, and 81901947) and the Basic Scientific Research of Central University Fund (2042019kf0056).
Author information
Authors and Affiliations
Contributions
Zhongyuan Xia designed the overall project and supervised the study; Xiazhong Yuan, Yelong Ji, Yang Wu, and Yan Leng designed the experiments; Yelong Ji and Zhen Qiu performed in vivo experiments; Yelong Ji and Yi Zhang performed in vitro experiment; Yelong Ji, Hao Ming, and Aining Zhang performed formal analysis; Yelong Ji, Yang Wu, and Yan Leng wrote the paper with input from other authors. Zhongyuan Xia and Shaoqing Lei reviewed and supervised the paper. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ji, Y., Leng, Y., Lei, S. et al. The mitochondria-targeted antioxidant MitoQ ameliorates myocardial ischemia–reperfusion injury by enhancing PINK1/Parkin-mediated mitophagy in type 2 diabetic rats. Cell Stress and Chaperones 27, 353–367 (2022). https://doi.org/10.1007/s12192-022-01273-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-022-01273-1