Abstract
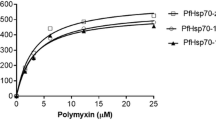
Plasmodium falciparum is the most lethal malaria parasite. The present study investigates the interaction capabilities of select plant derivatives, iso-mukaadial acetate (IMA) and ursolic acid acetate (UAA), against P. falciparum Hsp70-1 (PfHsp70-1) using in vitro approaches. PfHsp70-1 facilitates protein folding in the parasite and is deemed a prospective antimalarial drug target. Recombinant PfHsp70-1 protein was expressed in E. coli BL21 cells and homogeneously purified by affinity chromatography. The interaction between the compounds and PfHsp70-1 was evaluated using malate dehydrogenase (MDH), and luciferase aggregation assay, ATPase activity assay, and Fourier transform infrared (FTIR). PfHsp70-1 prevented the heat-induced aggregation of MDH and luciferase. However, the PfHsp70-1 chaperone role was inhibited by IMA or UAA, leading to both MDH and luciferase’s thermal aggregation. The basal ATPase activity of PfHsp70-1 (0.121 nmol/min/mg) was closer to UAA (0.131 nmol/min/mg) (p = 0.0675) at 5 mM compound concentration, suggesting that UAA has no effect on PfHsp70-1 ATPase activity. However, ATPase activity inhibition was similar between IMA (0.068 nmol/min/mg) (p < 0.0001) and polymyxin B (0.083 nmol/min/mg) (p < 0.0001). The lesser the Pi values, the lesser ATP hydrolysis observed due to compound binding to the ATPase domain. FTIR spectra analysis of IMA and UAA resulted in PfHsp70-1 structural alteration for β-sheets shifting the amide I band from 1637 cm−1 to 1639 cm−1, and for α-helix from 1650 cm−1 to 1652 cm−1, therefore depicting secondary structural changes with an increase in secondary structure percentage suggesting that these compounds interact with PfHsp70-1.





Similar content being viewed by others
References
Alhazmi HA (2019) FT-IR spectroscopy for the identification of binding sites and measurements of the binding interactions of important metal ions with bovine serum albumin. Sci Pharm. https://doi.org/10.3390/scipharm87010005
Ayeni G, Pooe OJ, Singh M et al (2019) Cytotoxic and antioxidant activities of selected South African medicinal plants. Pharmacogn J:11. https://doi.org/10.5530/PJ.2019.11.234
Bekono BD, Ntie-Kang F, Onguéné PA, et al (2020) The potential of anti-malarial compounds derived from African medicinal plants: a review of pharmacological evaluations from 2013 to 2019. Malar. J.
Botha M, Pesce E-R, Blatch GL (2007) The Hsp40 proteins of Plasmodium falciparum and other apicomplexa: regulating chaperone power in the parasite and the host. Int J Biochem Cell Biol 39:1781–1803. https://doi.org/10.1016/j.biocel.2007.02.011
Calabrese V, Cornelius C, Dinkova-Kostova AT et al (2010) Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxidants Redox Signal 13:1763–1811. https://doi.org/10.1089/ars.2009.3074
Calabrese V, Cornelius C, Cuzzocrea S et al (2011) Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Aspects Med 32:279–304. https://doi.org/10.1016/j.mam.2011.10.007
Cock IE, Selesho MI, van Vuuren SF (2019) A review of the traditional use of southern African medicinal plants for the treatment of malaria. J Ethnopharmacol 245:112176. https://doi.org/10.1016/J.JEP.2019.112176
Daniyan MO, Przyborski JM, Shonhai A (2019) Partners in mischief: functional networks of heat shock proteins of Plasmodium falciparum and their influence on parasite virulence. Biomolecules 9:295. https://doi.org/10.3390/biom9070295
Doyle SM, Hoskins JR, Kravats AN et al (2019) Intermolecular interactions between Hsp90 and Hsp70. J Mol Biol 431:2729–2746. https://doi.org/10.1016/J.JMB.2019.05.026
Hands JR, Clemens G, Stables R et al (2016) Brain tumour differentiation: rapid stratified serum diagnostics via attenuated total reflection Fourier-transform infrared spectroscopy. J Neurooncol. https://doi.org/10.1007/s11060-016-2060-x
Kampinga HH, Craig EA (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11:579–592. https://doi.org/10.1038/nrm2941
Kayamba F, Malimabe T, Kehinde I et al (2021) European Journal of Medicinal Chemistry Design and synthesis of quinoline-pyrimidine inspired hybrids as potential plasmodial inhibitors. Eur J Med Chem 217:113330. https://doi.org/10.1016/j.ejmech.2021.113330
Kong J, Yu S (2007) Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin (Shanghai) 39:549–559. https://doi.org/10.1111/j.1745-7270.2007.00320.x
Kyaw A, Maung-U K, Toe T (1985) Determination of inorganic phosphate with molybdate and Triton X-100 without reduction. Anal Biochem. https://doi.org/10.1016/0003-2697(85)90354-9
Litvinov RI, Faizullin DA, Zuev YF, Weisel JW (2012) The α-helix to β-sheet transition in stretched and compressed hydrated fibrin clots. Biophys J. https://doi.org/10.1016/j.bpj.2012.07.046
Mabate B, Zininga T, Ramatsui L et al (2018) Structural and biochemical characterization of Plasmodium falciparum Hsp70-x reveals functional versatility of its C-terminal EEVN motif. Proteins Struct Funct Bioinforma. https://doi.org/10.1002/prot.25600
Makhoba XH, Viegas C, Mosa RA et al (2020) Potential impact of the multi-target drug approach in the treatment of some complex diseases. Drug Des Devel Ther 14:3235–3249. https://doi.org/10.2147/DDDT.S257494
Matambo TS, Odunuga OO, Boshoff A, Blatch GL (2004) Overproduction, purification, and characterization of the Plasmodium falciparum heat shock protein 70. Protein Expr Purif. https://doi.org/10.1016/j.pep.2003.09.010
Mattson MP (2008) Hormesis defined. Ageing Res Rev 7:1–7. https://doi.org/10.1016/j.arr.2007.08.007
Miller DJ, Fort PE (2018) Heat shock proteins regulatory role in neurodevelopment. Front. Neurosci.
Misra G, Ramachandran R (2009) Hsp70-1 from Plasmodium falciparum: protein stability, domain analysis and chaperone activity. Biophys Chem. https://doi.org/10.1016/j.bpc.2009.03.006
Nyaba ZN, Murambiwa P, Opoku AR et al (2018) Isolation, characterization, and biological evaluation of a potent anti-malarial drimane sesquiterpene from Warburgia salutaris stem bark. Malar J 17:1–8. https://doi.org/10.1186/s12936-018-2439-6
Opoku F, Govender PP, Pooe OJ, Simelane MBC (2019) Evaluating iso-mukaadial acetate and ursolic acid acetate as plasmodium falciparum hypoxanthineguanine-xanthine phosphoribosyltransferase inhibitors. Biomolecules. https://doi.org/10.3390/biom9120861
Pooe OJ, Köllisch G, Heine H, Shonhai A (2017) Plasmodium falciparum heat shock protein 70 lacks immune modulatory activity. Protein Pept Lett 24:503–510. https://doi.org/10.2174/0929866524666170214141909
Przyborski JM, Diehl M, Blatch GL (2015) Plasmodial HSP70s are functionally adapted to the malaria parasite life cycle. Front Mol Biosci 2:34. https://doi.org/10.3389/fmolb.2015.00034
Shonhai A (2014) The role of Hsp70s in the development and pathogenicity of plasmodium species. In: Heat Shock Proteins of Malaria
Shonhai A, Boshoff A, Blatch GL (2007) The structural and functional diversity of Hsp70 proteins from Plasmodium falciparum. Protein Sci. https://doi.org/10.1110/ps.072918107
Shonhai A, Botha M, de Beer T et al (2008) Structure-function study of a Plasmodium falciparum Hsp70 using three dimensional modelling and in vitro analyses. Protein Pept Lett. https://doi.org/10.2174/092986608786071067
Simelane M, Shonhai A, Shode F et al (2013) Anti-plasmodial activity of some zulu medicinal plants and of some triterpenes isolated from them. Molecules 18:12313–12323. https://doi.org/10.3390/molecules181012313
Siracusa R, Scuto M, Fusco R et al (2020) Anti-inflammatory and anti-oxidant activity of hidrox® in rotenone-induced Parkinson’s disease in mice. Antioxidants 9:1–19. https://doi.org/10.3390/antiox9090824
Stephens LL, Shonhai A, Blatch GL (2011) Co-expression of the Plasmodium falciparum molecular chaperone, PfHsp70, improves the heterologous production of the antimalarial drug target GTP cyclohydrolase I, PfGCHI. Protein Expr Purif 77:159–165. https://doi.org/10.1016/j.pep.2011.01.005
Xu X, Sarbeng EB, Vorvis C et al (2012) Unique peptide substrate binding properties of 110-kDa heat-shock protein (Hsp110) determine its distinct chaperone activity. J Biol Chem 287:5661. https://doi.org/10.1074/JBC.M111.275057
Zininga T, Shonhai A, Zininga T, Shonhai A (2014) Are heat shock proteins druggable candidates? Am J Biochem Biotechnol 10:208–210. https://doi.org/10.3844/ajbbsp.2014.208.210
Zininga T, Makumire S, Gitau GW et al (2015) Plasmodium falciparum hop (PfHop) interacts with the Hsp70 chaperone in a nucleotide-dependent fashion and exhibits ligand selectivity. PLoS One 10:e0135326. https://doi.org/10.1371/journal.pone.0135326
Zininga T, Pooe OJ, Makhado PB et al (2017) Polymyxin B inhibits the chaperone activity of Plasmodium falciparum Hsp70. Cell Stress Chaperones. https://doi.org/10.1007/s12192-017-0797-6
Funding
The authors would like to appreciate University of Johannesburg research fund (URC) (2021URC00229).
Author information
Authors and Affiliations
Contributions
Writing of the original draft; N.S, O.J.P., M.B.C.S.; data analysis; N.S.,O.J.P., M.B.C.S.; investigation; N.S., O.J.P., M.B.C.S.; supervision; M.B.C.S.,; funding acquisition; M.B.C.S. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 411 kb)
Rights and permissions
About this article
Cite this article
Salomane, N., Pooe, O.J. & Simelane, M.B.C. Iso-mukaadial acetate and ursolic acid acetate inhibit the chaperone activity of Plasmodium falciparum heat shock protein 70-1. Cell Stress and Chaperones 26, 685–693 (2021). https://doi.org/10.1007/s12192-021-01212-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-021-01212-6