Abstract
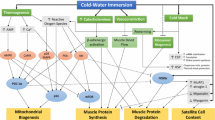
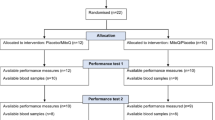
This study aims to evaluate the effect of regular post-exercise cold water immersion (CWI) on intramuscular markers of cellular stress response and signaling molecules related to mitochondria biogenesis and exercise performance after 4 weeks of high intensity interval training (HIIT). Seventeen healthy subjects were allocated into two groups: control (CON, n = 9) or CWI (n = 8). Each HIIT session consisted of 8–12 cycling exercise stimuli (90–110 % of peak power) for 60 s followed by 75 s of active recovery three times per week, for 4 weeks (12 HIIT sessions). After each HIIT session, the CWI had their lower limbs immersed in cold water (10 °C) for 15 min and the CON recovered at room temperature. Exercise performance was evaluated before and after HIIT by a 15-km cycling time trial. Vastus lateralis biopsies were obtained pre and 72 h post training. Samples were analyzed for heat shock protein 72 kDa (Hsp72), adenosine monophosphate-activated protein kinase (AMPK), and phosphorylated p38 mitogen-activated protein kinase (p-p38 MAPK) assessed by western blot. In addition, the mRNA expression of heat shock factor-1 (HSF-1), peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α), nuclear respiratory factor 1 and 2 (NRF1 and 2), mitochondrial transcription factor A (Tfam), calcium calmodulin-dependent protein kinase 2 (CaMK2) and enzymes citrate synthase (CS), carnitine palmitoyltransferase I (CPT1), and pyruvate dehydrogenase kinase (PDK4) were assessed by real-time PCR. Time to complete the 15-km cycling time trial was reduced with training (p < 0.001), but was not different between groups (p = 0.33). The Hsp72 (p = 0.01), p38 MAPK, and AMPK (p = 0.04) contents increased with training, but were not different between groups (p > 0.05). No differences were observed with training or condition for mRNA expression of PGC-1α (p = 0.31), CPT1 (p = 0.14), CS (p = 0.44), and NRF-2 (p = 0.82). However, HFS-1 (p = 0.007), PDK4 (p = 0.03), and Tfam (p = 0.03) mRNA were higher in CWI. NRF-1 decrease in both groups after training (p = 0.006). CaMK2 decreased with HIIT (p = 0.003) but it was not affected by CWI (p = 0.99). Cold water immersion does not alter HIIT-induced Hsp72, AMPK, p38 MAPK, and exercise performance but was able to increase some markers of cellular stress response and signaling molecules related to mitochondria biogenesis.




Similar content being viewed by others
References
ACSM (2014) In: Linda S Pescatello, Ross Arena, Deborah Riebe, Paul D Thompson (eds). ACSM’s Guidelines for Exercise Testing and Prescription. 9th Ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins
Bailey DM, Erith SJ, Griffin PJ, Dowson A, Brewer DS, Gant N, Williams C (2007) Influence of cold-water immersion on indices of muscle damage following prolonged intermittent shuttle running. J Sports Sci 25:1163–1170
Bergström J, Hultman E (1966) Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature 210:309–310
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Broatch JR, Petersen A, Bishop DJ (2014) Postexercise cold-water immersion benefits are not greater than the placebo effect. Med Sci Sports Exerc 46:2139–2147
Bruton JD, Aydin J, Yamada T, Shabalina IG, Ivarsson N, Zhang SJ, Wada M, Tavi P, Nedergaard J, Katz A, Westerblad H (2010) Increased fatigue resistance linked to Ca2+-stimulated mitochondrial biogenesis in muscle fibres of cold-acclimated mice. J Physiol 588(Pt 21):4275–4288. doi:10.1113/jphysiol.2010.198598
Buck MJ, Squire TL, Andrews MT (2002) Coordinate expression of the PDK4 gene: a means of regulating fuel selection in a hibernating mammal. Physiol Genomics 8(1):5–13
Burgomaster KA, Heigenhauser GJ, Gibala MJ (2006) Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J Appl Physiol 100:2041–2047
Crampton D, Donne B, Warmington SA, Egaña M (2013) Cycling time to failure is better maintained by cold than contrast or thermoneutral lower-body water immersion in normothermia. Eur J Appl Physiol 113:3059–3067
De Matos MA, de Oliveira Ottone V, Duarte TC, Sampaio PF, Costa KB, Fonseca CA, Neves MP, Schneider SM, Moseley P, Coimbra CC, Magalhães FC, Rocha-Vieira E, Amorim FT (2014) Exercise reduces cellular stress related to skeletal muscle insulin resistance. Cell Stress Chaperones 19:263–270
Dunne A, Crampton D, Egaña M (2013) Effect of post-exercise hydrotherapy water temperature on subsequent exhaustive running performance in normothermic conditions. J Sci Med Sport 16:466–471
Guttman SD, Glover CV, Allis CD, Gorovsky MA (1980) Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell 22:299–307
Haddad HA, Parouty J, Buchheit M (2012) Effect of daily cold water immersion on heart rate variability and subjective ratings of well-being in highly trained swimmers. Int J Sports Physiol Perform 7:33–38
Halson SL, Bartram J, West N, Stephens J, Argus CK, Driller MW, Sargent C, Lastella M, Hopkins WG, Martin DT (2014) Does hydrotherapy help or hinder adaptation to training in competitive cyclists. Med Sci Sports Exerc 46:1631–1639
Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, Chung J, Watson N, Gardner T, Lee-Young RS, Connor T, Watt MJ, Carpenter K, Hargreaves M, McGee SL, Hevener AL, Febbraio MA (2014) Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 63:1881–1894
Ihsan M, Watson G, Lipski M, Abbiss CR (2013) Influence of postexercise cooling on muscle oxygenation and blood volume changes. Med Sci Sports Exerc 45:876–882
Ihsan M, Watson G, Choo HC, Lewandowski P, Papazzo A, Cameron-Smith D, Abbiss CR (2014) Postexercise muscle cooling enhances gene expression of PGC-1α. Med Sci Sports Exerc 46(10):1900–1907
Ihsan M, Markworth JF, Watson G, Choo HC, Govus A, Pham T, Hickey A, Cameron-Smith D, Abbiss CR (2015) Regular postexercise cooling enhances mitochondrial biogenesis through AMPK and p38 MAPK in human skeletal muscle. Am J Physiol-Regul Integr Comp Physiol 309:R286–R294
Kramer HF, Goodyear LJ (2007) Exercise, MAPK, and NF-kB signaling in skeletal muscle. J Appl Physiol 103:388–395
Kregel KC (2002) Invited review: heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92:2177–2186
Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ (2010) A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588:1011–1022
Liu Y, Mayr S, Opitz-Gress A, Zeller C, Lormes W, Baur S, Lehmann M, Steinacker JM (1999) Human skeletal muscle HSP70 response to training in highly trained rowers. J Appl Physiol 86:101–104
Liu CC, Lin CH, Lee CC, Lin MT, Wen HC (2013) Transgenic overexpression of heat shock protein 72 in mouse muscle protects against exhaustive exercise-induced skeletal muscle damage. J Form Med Assoc 112:24e30
Livak, K. J.; Schmittgen, T. D. Analysis of relative gene expression data using real time quantitative PCR and the 2-(ΔCT) method. Methods, v. 25, n. 4, p. 402-8, DEC, 2001.
Locke M, Atkinson BG, Tanguay RM, Noble EG (1994) Shifts in type I fiber proportion in rat hindlimb muscle are accompanied by changes in HSP72 content. Am J Physiol-Cell Physiol 266:C1240–C1246
Mawhinney C, Jones H, Joo CH, Low DA, Green DJ, Gregson W (2013) Influence of cold-water immersion on limb and cutaneous blood flow after exercise. Med Sci Sports Exerc 45:2277–2285
Miller BF, Konopka AR, Hamilton KL. (2016) The rigorous study of exercise adaptations: why mRNA might not be enough. J Appl Physiol (1985). Mar 24:jap.00137.2016. doi: 10.1152/japplphysiol.00137
Peiffer JJ, Abbiss CR, Watson G, Nosaka K, Laursen PB (2009) Effect of cold-water immersion duration on body temperature and muscle function. J Sports Sci 27:987–993
Perry CG, Lally J, Holloway G, Heigenhauser GJ, Bonen A, Spriet LL (2010) Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588(Pt 23):4795–4810. doi: 10.1113/jphysiol.2010.199448
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839
Reid MB (2005) Response of the ubiquitin-proteasome pathway to changes in muscle activity. Am J Physiol-Regul Integr Comp Physiol 288:R1423–R1431
Reznick RM, Shulman GI (2006) The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol 574:33–39
Roberts LA, Nosaka K, Coombes JS, Peake JM (2014) Cold water immersion enhances recovery of submaximal muscle function after resistance exercise. Am J Physiol-Regul Integr Comp Physiol 307:R998–R1008
Rowsell GJ, Reaburn P, Toone R, Smith M, Coutts AJ (2014) Effect of run training and cold-water immersion on subsequent cycle training quality in high-performance triathletes. J Strength Cond Res 28:1664–1672
Sciandra JJ, Subjeck JR (1983) The effects of glucose on protein synthesis and thermosensitivity in Chinese hamster ovary cells. J Biol Chem 258:12091–12093
Sonna LA, Fujita J, Gaffin SL, Lilly CM (2002) Effects of heat and cold stress on mammalian gene expression. J Appl Physiol 92:1725–1742. doi:10.1152/japplphysiol.01143.2001
Stanley J, Peake JM, Coombes JS, Buchheit M (2014) Central and peripheral adjustments during high-intensity exercise following cold water immersion. Eur J Appl Physiol 114:147–163
Takahashi M, Chesley A, Freyssenet D, Hood DA (1998) Contractile activity-induced adaptations in the mitochondrial protein import system. Am J Physiol-Cell Physiol 274:C1380–C1387
Tupling AR, Gramolini AO, Duhamel TA, Kondo H, Asahi M, Tsuchiya SC, Borrelli MJ, Lepock JR, Otsu K, Hori M, MacLennan DH, Green HJ (2004) HSP70 binds to the fast-twitch skeletal muscle sarco (endo) plasmic reticulum Ca2+-ATPase (SERCA1a) and prevents thermal inactivation. J Biol Chem 279:52382–52389
Vaile J, Halson S, Gill N, Dawson B (2008) Effect of hydrotherapy on the signs and symptoms of delayed onset muscle soreness. Eur J Appl Physiol 102:447–455
Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol., v. 3, n. 7, JUN, 2002.
Versey NG, Halson SL, Dawson BT (2013) Water immersion recovery for athletes: effect on exercise performance and practical recommendations. Sports Med 43:1101–1130
Vogt M, Puntschart A, Geiser J, Zuleger C, Billeter R, Hoppeler H (2001) Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J Appl Physiol 91:173–182
Wende AR, Huss JM, Schaeffer PJ, Giguère V, Kelly DP (2005) PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol 25(24):10684–10694
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124
Yamane M, Teruya H, Nakano M, Ogai R, Ohnishi N, Kosaka M (2006) Post-exercise leg and forearm flexor muscle cooling in humans attenuates endurance and resistance training effects on muscle performance and on circulatory adaptation. Eur J Appl Physiol 96:572–580
Yamane M, Ohnishi N, Matsumoto T (2015) Does regular post-exercise cold application attenuate trained muscle adaptation? Int J Sports Med 36(8):647–653
Acknowledgments
The authors wish to acknowledge Dr. Miguel Proença for performing the muscle biopsy and all the volunteers that participated in the present study. This work was supported by CAPES (PNPD-2455/2011), FAPEMIG (APQ-01382-12), and CNPq (407252/2013-4 and 445096/2014-4) grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguiar, P.F., Magalhães, S.M., Fonseca, I.A.T. et al. Post-exercise cold water immersion does not alter high intensity interval training-induced exercise performance and Hsp72 responses, but enhances mitochondrial markers. Cell Stress and Chaperones 21, 793–804 (2016). https://doi.org/10.1007/s12192-016-0704-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-016-0704-6