Abstract
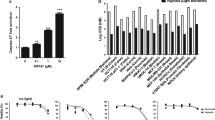
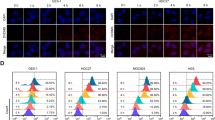
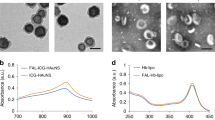
Photodynamic therapy (PDT) is a recently developed antitumor modality utilizing the generation of reactive oxygen species (ROS), through light irradiation of photosensitizers (PSs) localized in tumor. Interference with proper functioning of endoplasmic reticulum (ER) by ER-targeting PDT is a newly proposed strategy to achieve tumor cell death. The aim of this study is to establish a multifunctional model to screen and assess ER-targeting PSs based on luciferase reporters system. Upregulation of GRP78 is a biomarker for the onset of ER stress. CHOP is a key initiating player in ER stress-induced cell death. Here, the most sensitive fragments of GRP78 and CHOP promoters responding to ER-targeting PDT were mapped and cloned into pGL3-basic vector, forming −702/GRP78-Luc and −443/CHOP-Luc construct, respectively. We demonstrated that −702/GRP78-Luc expression can be used to indicate the ER-targeting of PSs, meanwhile estimate the ROS level induced by low-dose ER-targeting PDT. Moreover, the luciferase signaling of −443/CHOP-Luc showed highly consistence with apoptosis rate caused by ER-targeting PDT, suggesting that −443/CHOP-Luc can evaluate the antitumor properties of PSs. Hypericin, Foscan® and methylene blue were applied to verify the sensitivity and reliability of our model. These results proved that GRP78-CHOP model may be suitable to screen ER-targeting photosensitive compounds with lower cost and higher sensitivity than traditional ways.






Similar content being viewed by others
References
Ali SM, Olivo M (2002) Bio-distribution and subcellular localization of Hypericin and its role in PDT induced apoptosis in cancer cells. Int J Oncol 21(3):531–540
Boyce M, Yuan J (2006) Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ 13(3):363–373
Buytaert E, Matroule J, Durinck S, Close P, Kocanova S, Vandenheede J, De Witte P, Piette J, Agostinis P (2007) Molecular effectors and modulators of hypericin-mediated cell death in bladder cancer cells. Oncogene 27(13):1916–1929
Castano AP, Demidova TN, Hamblin MR (2004) Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodynamic Ther 1(4):279–293
Castano AP, Demidova TN, Hamblin MR (2005) Mechanisms in photodynamic therapy: part two—cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodynamic Ther 2(1):1–23
Cheng X, Guerasimova A, Manke T, Rosenstiel P, Haas S, Warnatz H-J, Querfurth R, Nietfeld W, Vanhecke D, Lehrach H (2010) Screening of human gene promoter activities using transfected-cell arrays. Gene 450(1):48–54
Davids LM, Kleemann B, Kacerovská D, Pizinger K, Kidson SH (2008) Hypericin phototoxicity induces different modes of cell death in melanoma and human skin cells. J Photochem Photobiol, B 91(2):67–76
Dolmans DE, Fukumura D, Jain RK (2003) Photodynamic therapy for cancer. Nat Rev Cancer 3(5):380–387
Dudek J, Benedix J, Cappel S, Greiner M, Jalal C, Müller L, Zimmermann R (2009) Functions and pathologies of BiP and its interaction partners. Cell Mol Life Sci 66(9):1556–1569
François A, Marchal S, Guillemin F, Bezdetnaya L (2011) mTHPC-based photodynamic therapy induction of autophagy and apoptosis in cultured cells in relation to mitochondria and endoplasmic reticulum stress. Int J Oncol 39(6):1537–1543
Fuchs J, Weber S, Kaufmann R (2000) Genotoxic potential of porphyrin type photosensitizers with particular emphasis on 5-aminolevulinic acid: implications for clinical photodynamic therapy. Free Radical Biol Med 28(4):537–548
Gabrielli D, Belisle E, Severino D, Kowaltowski AJ, Baptista MS (2004) Binding, aggregation and photochemical properties of methylene blue in mitochondrial suspensions. Photochem Photobiol 79(3):227–232
Gomer CJ, Ferrario A, Rucker N, Wong S, Lee AS (1991) Glucose regulated protein induction and cellular resistance to oxidative stress mediated by porphyrin photosensitization. Cancer Res 51(24):6574–6579
Gomer CJ, Ryter SW, Ferrario A, Rucker N, Wong S, Fisher AM (1996) Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res 56(10):2355–2360
Grebeňová D, Kuželová K, Smetana K, Pluskalová M, Cajthamlová H, Marinov I, Fuchs O, Souček J, Jarolim P, Hrkal Z (2003) Mitochondrial and endoplasmic reticulum stress-induced apoptotic pathways are activated by 5-aminolevulinic acid-based photodynamic therapy in HL60 leukemia cells. J Photochem Photobiol, B 69(2):71–85
Guyton K, Xu Q, Holbrook N (1996) Induction of the mammalian stress response gene GADD153 by oxidative stress: role of AP-1 element. Biochem J 314:547–554
Korbelik M, Sun J, Cecic I (2005) Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res 65(3):1018–1026
Kwok SC, Daskal I (2008) Brefeldin A activates CHOP promoter at the AARE, ERSE and AP-1 elements. Mol Cell Biochem 319(1–2):203–208
Lee AS (2005) The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35(4):373–381
Li J, Lee AS (2006) Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med 6(1):45–54
Li B, Chu X, Gao M, Li W (2010) Apoptotic mechanism of MCF-7 breast cells in vivo and in vitro induced by photodynamic therapy with C-phycocyanin. Acta Biochim Biophys Sin 42(1):80–89
Liu RY, Kim D, Yang S-H, Li GC (1993) Dual control of heat shock response: involvement of a constitutive heat shock element-binding factor. Proc Natl Acad Sci U S A 90(7):3078–3082
Logue SE, Cleary P, Saveljeva S, Samali A (2013) New directions in ER stress-induced cell death. Apoptosis 18(5):537–46
Luna MC, Ferrario A, Wong S, Fisher AM, Gomer CJ (2000) Photodynamic therapy-mediated oxidative stress as a molecular switch for the temporal expression of genes ligated to the human heat shock promoter. Cancer Res 60(6):1637–1644
L-y X, S-m C, Oleinick NL (2001) Photodynamic therapy-induced death of MCF-7 human breast cancer cells: a role for caspase-3 in the late steps of apoptosis but not for the critical lethal event. Exp Cell Res 263(1):145–155
Marchal S, François A, Dumas D, Guillemin F, Bezdetnaya L (2007) Relationship between subcellular localisation of Foscan® and caspase activation in photosensitised MCF-7 cells. Br J Cancer 96(6):944–951
Moserova I, Kralova J (2012) Role of ER stress response in photodynamic therapy: ROS generated in different subcellular compartments trigger diverse cell death pathways. PLoS One 7(3):e32972
Oyadomari S, Mori M (2003) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11(4):381–389
Peng Q, Moan J, Nesland JM (1996) Correlation of subcellular and intratumoral photosensitizer localization with ultrastructural features after photodynamic therapy. Ultrastruct Pathol 20(2):109–129
Radak Z, Chung HY, Goto S (2005) Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology 6(1):71–75
Ritz R, Roser F, Radomski N, Strauss WS, Tatagiba M, Gharabaghi A (2008) Subcellular colocalization of hypericin with respect to endoplasmic reticulum and Golgi apparatus in glioblastoma cells. Anticancer Res 28(4B):2033–2038
Robertson C, Evans DH, Abrahamse H (2009) Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol, B 96(1):1–8
Schönthal AH (2013) Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem Pharmacol 85(5):653–666
Shen K, Zhang H, Sun L, Xu Y, Qian X, Liu J (2013) A ROS-mediated lysosomal–mitochondrial pathway is induced by a novel Amonafide analogue, 7c, in human Hela cervix carcinoma cells. Cancer Lett 333(2):229–238
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7(9):880–885
Szokalska A, Makowski M, Nowis D, Wilczyński GM, Kujawa M, Wójcik C, Młynarczuk-Biały I, Salwa P, Bil J, Janowska S (2009) Proteasome inhibition potentiates antitumor effects of photodynamic therapy in mice through induction of endoplasmic reticulum stress and unfolded protein response. Cancer Res 69(10):4235–4243
Teiten M, Bezdetnaya L, Morliere P, Santus R, Guillemin F (2003) Endoplasmic reticulum and Golgi apparatus are the preferential sites of Foscan® localisation in cultured tumour cells. Br J Cancer 88(1):146–152
Walther W, Stein U (2009) Heat-responsive gene expression for gene therapy. Adv Drug Delivery Rev 61(7):641–649
Wong S, Luna M, Ferrario A, Gomer CJ (2004) CHOP activation by photodynamic therapy increases treatment induced photosensitization. Lasers Surg Med 35(5):336–341
Woo KJ, Lee TJ, Lee SH, Lee J-M, Seo J-H, Jeong Y-J, Park J-W, Kwon TK (2007) Elevated gadd153/chop expression during resveratrol-induced apoptosis in human colon cancer cells. Biochem Pharmacol 73(1):68–76
Yoshida H, Haze K, Yanagi H, Yura T, Mori K (1998) Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins Involvement of basic leucine zipper transcription factors. J Biol Chem 273(50):33741–33749
Zheng Y, Le V, Cheng Z, Xie S, Li H, Tian J, Liu J (2013) Development of rapid and highly sensitive HSPA1A promoter-driven luciferase reporter system for assessing oxidative stress associated with low-dose photodynamic therapy. Cell Stress Chaperones 18(2):203–213
Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12(7):982–995
Acknowledgments
This work was supported by Shanghai Committee of Science and Technology (No. 13140902300), Nano Science and Technology Special Funding of Shanghai Committee of Science and Technology (No. 11 nm0503700), the Shanghai Committee of Science and Technology [grant 11DZ2260600] and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lin, S., Zhang, L., Lei, K. et al. Development of a multifunctional luciferase reporters system for assessing endoplasmic reticulum-targeting photosensitive compounds. Cell Stress and Chaperones 19, 927–937 (2014). https://doi.org/10.1007/s12192-014-0517-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-014-0517-4