Abstract
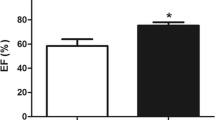
Tetralogy of Fallot (TOF) is a congenital heart condition in which the right ventricle is exposed to cyanosis and pressure overload. Patients have an increased risk of right ventricle dysfunction following corrective surgery. Whether the cyanotic myocardium is less tolerant of injury compared to non-cyanotic is unclear. Heat shock proteins (HSPs) protect against cellular stresses. The aim of this study was to examine HSP 27 expression in the right ventricle resected from TOF patients and determine its relationship with right ventricle function and clinical outcome. Ten cyanotic and ten non-cyanotic patients were studied. Western blotting was used to quantify HSP 27 in resected myocardium at (1) baseline (first 15 min of aortic cross clamp and closest representation of pre-operative status) and (2) after 15 min during ischemia until surgery was complete. The cyanotic group had significantly increased haematocrit, lower O2 saturation, thicker interventricular septal wall thickness and released more troponin-I on post-operative day 1 (p < 0.05). HSP 27 expression was significantly increased in the <15 min cyanotic compared to the <15 min non-cyanotic group (p = 0.03). In the cyanotic group, baseline HSP 27 expression also significantly correlated with oxygen extraction ratio (p = 0.028), post-operative basal septal velocity (p = 0.036) and mixed venous oxygen saturation (p = 0.02), markers of improved cardiac output/contraction. Increased HSP 27 expression and associated improved right ventricle function and systemic perfusion supports a cardio-protective effect of HSP 27 in cyanotic TOF.



Similar content being viewed by others
References
Aloy MT, Hadchity E, Bionda C, Diaz-Latoud C, Claude L, Rousson R, Arrigo AP, Rodriguez-Lafrasse CC (2008) Protective role of Hsp27 protein against gamma radiation-induced apoptosis and radiosensitization effects of Hsp27 gene silencing in different human tumor cells. Int J Radiat Oncol Biol Phys 70:543–553
Arrigo AP, Simon S, Gibert B, Kretz-Remy C, Nivon M, Czekalla A, Guillet D, Moulin M, Diaz-Latoud C, Vicart P (2007) Hsp27 (HspB1) and alphaB-crystallin (HspB5) as therapeutic targets. FEBS Lett 581:3665–3674
Brar BK, Stephanou A, Wagstaff MJ, Coffin RS, Marber MS, Engelmann G, Latchman DS (1999) Heat Shock Proteins Delivered With a Virus Vector Can Protect Cardiac Cells Against Apoptosis As Well As Against Thermal or Hypoxic Stress. J Mol Cardiol 1:135–146
Brown DD, Christine KS, Showell C, Conlon FL (2007) Small heat shock protein Hsp27 is required for proper heart tube formation. Genesis 45:667–768
Bulutcu FS, Bayindir O, Polat B, Yalcin Y, öZbek U, Cakali E (2002) Does normoxemic cardiopulmonary bypass prevent myocardial reoxygenation injury in cyanotic children? J Cardiothorac Vasc Anesth 16:330–333
Carper SW, Rocheleau TA, Cimino D, Storm FK (1997) Heat shock protein 27 stimulates recovery of RNA and protein synthesis following a heat shock. J Cell Biochem 66:153–164
Cullen S, Shore D, Redington A (1995) Characterization of right ventricular diastolic performance after complete repair of tetralogy of Fallot. Restrictive physiology predicts slow postoperative recovery. Circ 91:1782–1789
Davlouros PA, Niwa K, Webb G, Gatzoulis MA (2006) The right ventricle in congenital heart disease. Heart 92:27–28
Efthymiou CA, Mocanu MM, de Belleroche J, Wells DJ, Latchmann DS, Yellon DM (2004) Heat shock protein 27 protects the heart against myocardial infarction. Basic Res Cardiol 99:392–394
Eisen A, Rubenfire FM, Freimark D, McKechnie R, Tenenbaum A, Motro M, Adler Y (2004) Ischemic preconditioning: nearly two decades of research. A comprehensive review. Atherosclerosis 172:201–210
Franklin TB, Krueger-Naug AM, Clarke DB, Arrigo AP, Currie RW (2005) The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int J Hyperthermia 21:379–392
Ghayour-Mobarhan M, Saber H, Ferns GA (2012) The potential role of heat shock protein 27 in cardiovascular disease. Clin Chim Acta 413:15–24
Ghorbel MT, Cherif M, Jenkins E, Mokhtari A, Kenny D, Angelini GD, Caputo M (2010) Transcriptomic analysis of patients with tetralogy of Fallot reveals the effect of chronic hypoxia on myocardial gene expression. J Thorac Cardiovasc Surg 140:337–345
Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH (2004) Overexpression of wild–type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circ 110:3544–3552
Imura H, Caputo M, Parry A, Pawade A, Angelini GD, Suleiman MS (2001) Age-dependent and hypoxia-related differences in myocardial protection during pediatric open heart surgery. Circ 103:1551–1556
Jayakumar J, Suzuki K, Khan M, Smolenski RT, Farrell A, Latif N, Raisky O, Abunasra H, Sammut IA, Murtuza B, Amrani M, Yacoub MH (2000) Gene therapy for myocardial protection: transfection of donor hearts with heat shock protein 70 gene protects cardiac function against ischemia reperfusion injury. Circ 102:III302–III306
Knowlton AA, Kapadia S, Torre-Amione G, Durand JB, Bies R, Young J, Mann DL (1998) Differential expression of heat shock proteins in normal and failing human hearts. J Mol Cell Cardiol 30:811–818
Lyall F, Barber A, Myatt L, Bulmer JN, Robson SC (2000) Hemeoxygenase expression in human placenta and placental bed implies a role in regulation of trophoblast invasion and placental function. FASEB J 14:208–219
Martin J, Shekerdemian LS (2009) The monitoring of venous saturations of oxygen in children with congenitally malformed hearts. Cardiol Young 19:34–39
Modi P, Imura H, Caputo M, Pawade A, Parry A, Angelini GD, Suleiman MS (2002) Cardiopulmonary bypass-induced myocardial reoxygenation injury in pediatric patients with cyanosis. J Thorac Cardiovasc Surg 124:1035–1036
Nakamura K, Irie H, Fujisawa E, Yoshioka H, Ninomiya Y, Sakuma I, Sano S (2000) Heat shock protein 72 expression in the right ventricle of patients undergoing congenital cardiac surgery. Acta Med Okayama 54:103–109
Nollert G, Fischlein T, Bouterwek S, Böhmer C, Klinner W, Reichart B (1997) Long-term survival in patients with repair of tetralogy of Fallot: 36-year follow-up of 490 survivors of the first year after surgical repair. J Am Coll Cardiol 30:1374–1383
Peng EW, McCaig D, Pollock JC, MacArthur K, Lyall F, Danton MH (2011) Myocardial expression of heat shock protein 70i protects early postoperative right ventricular function in cyanotic tetralogy of Fallot. J Thorac Cardiovasc Surg 141:1184–1191
Schmitt JP, Schunkert H, Birnbaum DE, Aebert H (2002) Kinetics of heat shock protein 70 synthesis in the human heart after cold cardioplegic arrest. Eur J Cardiothorac Surg 22:415–420
Starr JO (2010) Tetraology of Fallot: yesterday and today. World J Surg 34:658–668
Taggart DP, Bakkenist CJ, Biddolph SC, Graham AK, McGee JO (1997) Induction of myocardial heat shock protein 70 during cardiac surgery. J Pathol 182:362–366
VanderHeide RS (2002) Increased expression of HSP27 protects canine myocytes from simulated ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 282:H935–H941
Wernovsky G, Wypij D, Jonas RA, Mayer JE Jr, Hanley FL, Hickey PR, Walsh AZ, Chang AC, Castañeda AR, Newburger JW, Wessel DL (1995) Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circ 92:2226–2235
Willis MS, Patterson C (2010) Hold me tight: role of the heat shock protein family of chaperones in cardiac disease. Circ 122:1740–1751
Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee ME, Perrella MA (2001) Cardiac–specific expression of heme oxygenase–1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res 89:168–173
Acknowledgments
This work was supported by Yorkhill Children's Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walker, S., Danton, M., Peng, E.W.K. et al. Heat shock protein 27 is increased in cyanotic tetralogy of Fallot myocardium and is associated with improved cardiac output and contraction. Cell Stress and Chaperones 18, 269–277 (2013). https://doi.org/10.1007/s12192-012-0379-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-012-0379-6