Abstract
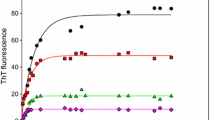
Amyloid fibril formation is associated with diseases such as Alzheimer’s, Parkinson’s, and prion diseases. Inhibition of amyloid fibril formation by molecular chaperone proteins, such as the small heat-shock protein αB-crystallin, may play a protective role in preventing the toxicity associated with this form of protein misfolding. Reduced and carboxymethylated κ-casein (RCMκ-CN), a protein derived from milk, readily and reproducibly forms fibrils at physiological temperature and pH. We investigated the toxicity of fibril formation by RCMκ-CN using neuronal model PC12 cells and determined whether the inhibition of fibril formation altered its cell toxicity. To resolve ambiguities in the literature, we also investigated whether fibril formation by amyloid-β1–40 (Aβ1–40), the peptide associated with Alzheimer’s disease, was inhibited by αB-crystallin and if this affected the toxicity of Aβ. To this end, either RCMκ-CN or Aβ1–40 was incubated at neutral pH to induce fibril formation before treating PC12 cells and assessing cell viability. Incubated (fibrillar) RCMκ-CN was more toxic to PC12 cells than native RCMκ-CN with the highest level of toxicity being associated with mature fibrils and protofibrils. Furthermore, the toxicity of RCMκ-CN was attenuated when its fibril formation was inhibited, either through the chaperone action of αB-crystallin or when it interacted with its natural binding partners in milk, αS- and β-casein. Likewise, incubating Aβ1–40 with αB-crystallin inhibited both Aβ1–40 fibril formation and the associated cell toxicity. Importantly, by inhibiting fibril formation, αB-crystallin prevents the cell toxicity associated with protein misfolding.








Similar content being viewed by others
Abbreviations
- Aβ:
-
amyloid-β
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- RCMκ-CN:
-
Reduced and carboxymethylated κ-casein
- sHsp:
-
small heat-shock protein
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- TEM:
-
transmission electron microscopy
- ThT:
-
Thioflavin T
References
Aquilina JA, Benesch JL, Ding LL, Yaron O, Horwitz J, Robinson CV (2004) Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J Biol Chem 279:28675–28680
Beems RB, Gruys E, Spit BJ (1978) Amyloid in the corpora amylacea of the rat mammary gland. Vet Pathol 15:347–352
Bodles AM, Guthrie DJ, Harriott P, Campbell P, Irvine GB (2000) Toxicity of non-abeta component of Alzheimer's disease amyloid, and N-terminal fragments thereof, correlates to formation of beta-sheet structure and fibrils. Eur J Biochem 267:2186–2194
Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M (2002) Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416:507–511
Carver JA, Rekas A, Thorn DC, Wilson MR (2003) Small heat-shock proteins and clusterin: intra- and extracellular molecular chaperones with a common mechanism of action and function? IUBMB Life 55:661–668
Carver JA, Duggan PJ, Ecroyd H, Liu Y, Meyer AG, Tranberg CE (2010) Carboxymethylated kappa-casein: a convenient tool for the identification of polyphenolic inhibitors of amyloid fibril formation. Bioorg Med Chem 18:222–228
Caughey B, Lansbury PT (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26:267–298
Chimon S, Shaibat MA, Jones CR, Calero DC, Aizezi B, Ishii Y (2007) Evidence of fibril-like beta-sheet structures in a neurotoxic amyloid intermediate of Alzheimer's beta-amyloid. Nat Struct Mol Biol 14:1157–1164
Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75:333–366
Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M (2007) Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci 27:9220–9232
Dobson CM (2004) Experimental investigation of protein folding and misfolding. Methods 34:4–14
Du HN, Tang L, Luo XY, Li HT, Hu J, Zhou JW, Hu HY (2003) A peptide motif consisting of glycine, alanine, and valine is required for the fibrillization and cytotoxicity of human alpha-synuclein. Biochemistry 42:8870–8878
Ecroyd H, Carver JA (2009) Crystallin proteins and amyloid fibrils. Cell Mol Life Sci 66:62–81
Ecroyd H, Meehan S, Horwitz J, Aquilina JA, Benesch JL, Robinson CV, Macphee CE, Carver JA (2007) Mimicking phosphorylation of alphaB-crystallin affects its chaperone activity. Biochem J 401:129–141
Ecroyd H, Koudelka T, Thorn DC, Williams DM, Devlin G, Hoffmann P, Carver JA (2008) Dissociation from the oligomeric state is the rate-limiting step in fibril formation by kappa-casein. J Biol Chem 283:9012–9022
El-Agnaf OM, Jakes R, Curran MD, Middleton D, Ingenito R, Bianchi E, Pessi A, Neill D, Wallace A (1998) Aggregates from mutant and wild-type alpha-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of beta-sheet and amyloid-like filaments. FEBS Lett 440:71–75
Farrell HM Jr, Cooke PH, Wickham ED, Piotrowski EG, Hoagland PD (2003) Environmental influences on bovine kappa-casein: reduction and conversion to fibrillar (amyloid) structures. J Protein Chem 22:259–273
Fox PF, McSweeney PLH (1998) Dairy Chemistry and Biochemistry. Blackie Academic and Professional, London, pp 150–169
Friedrich RP, Tepper K, Ronicke R, Soom M, Westermann M, Reymann K, Kaether C, Fandrich M (2010) Mechanism of amyloid plaque formation suggests an intracellular basis of Abeta pathogenicity. Proc Natl Acad Sci USA 107:1942–1947
Gruys E (2004) Protein misfolding in domestic animals. J Zhejiang Univ Sci 5:1226–1238
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 8:101–112
Hamley IW (2007) Peptide fibrillization. Angew Chem Int Ed Engl 46:8128–8147
Harper JD, Lansbury PT Jr (1997) Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem 66:385–407
Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ (1999) Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci 19:8876–8884
Hatters DM, Lindner RA, Carver JA, Howlett GJ (2001) The molecular chaperone, alpha-crystallin, inhibits amyloid formation by apolipoprotein C-II. J Biol Chem 276:33755–33761
Horwitz J, Huang QL, Ding L, Bova MP (1998) Lens alpha-crystallin: chaperone-like properties. Methods Enzymol 290:365–383
Hoshi M, Sato M, Matsumoto S, Noguchi A, Yasutake K, Yoshida N, Sato K (2003) Spherical aggregates of beta-amyloid (amylospheroid) show high neurotoxicity and activate tau protein kinase I/glycogen synthase kinase-3beta. Proc Natl Acad Sci USA 100:6370–6375
Lee S, Carson K, Rice-Ficht A, Good T (2005) Hsp20, a novel alpha-crystallin, prevents Abeta fibril formation and toxicity. Protein Sci 14:593–601
Lee S, Carson K, Rice-Ficht A, Good T (2006) Small heat shock proteins differentially affect Abeta aggregation and toxicity. Biochem Biophys Res Commun 347:527–533
Liang JJ (2000) Interaction between beta-amyloid and lens alphaB-crystallin. FEBS Lett 484:98–101
Lindner RA, Treweek TM, Carver JA (2001) The molecular chaperone alpha-crystallin is in kinetic competition with aggregation to stabilize a monomeric molten-globule form of alpha-lactalbumin. Biochem J 354:79–87
Lowe J, McDermott H, Pike I, Spendlove I, Landon M, Mayer RJ (1992) alpha B crystallin expression in non-lenticular tissues and selective presence in ubiquitinated inclusion bodies in human disease. J Pathol 166:61–68
Mattson MP (2006) Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid Redox Signal 8:1997–2006
McLean PJ, Kawamata H, Shariff S, Hewett J, Sharma N, Ueda K, Breakefield XO, Hyman BT (2002) TorsinA and heat shock proteins act as molecular chaperones: suppression of alpha-synuclein aggregation. J Neurochem 83:846–854
Morgan PE, Treweek TM, Lindner RA, Price WE, Carver JA (2005) Casein proteins as molecular chaperones. J Agric Food Chem 53:2670–2683
Nickerson SC (1987) Amyloid fibril formation in the bovine mammary gland: an ultrastructural study. Cytobios 51:81–92
Nickerson SC, Sordillo LM, Boddie NT, Saxton AM (1985) Prevalence and ultrastructural characteristics of bovine mammary corpora amylacea during the lactation cycle. J Dairy Sci 68:709–717
Novitskaya V, Bocharova OV, Bronstein I, Baskakov IV (2006) Amyloid fibrils of mammalian prion protein are highly toxic to cultured cells and primary neurons. J Biol Chem 281:13828–13836
O'Donovan CN, Tobin D, Cotter TG (2001) Prion protein fragment PrP-(106-126) induces apoptosis via mitochondrial disruption in human neuronal SH-SY5Y cells. J Biol Chem 276:43516–43523
Onoue S, Ohshima K, Endo K, Yajima T, Kashimoto K (2002) PACAP protects neuronal PC12 cells from the cytotoxicity of human prion protein fragment 106-126. FEBS Lett 522:65–70
Pountney DL, Treweek TM, Chataway T, Huang Y, Chegini F, Blumbergs PC, Raftery MJ, Gai WP (2005) Alpha B-crystallin is a major component of glial cytoplasmic inclusions in multiple system atrophy. Neurotox Res 7:77–85
Raman B, Ban T, Sakai M, Pasta SY, Ramakrishna T, Naiki H, Goto Y, Rao Ch M (2005) AlphaB-crystallin, a small heat-shock protein, prevents the amyloid fibril growth of an amyloid beta-peptide and beta2-microglobulin. Biochem J 392:573–581
Reid IM (1972) Corpora amylacea of the bovine mammary gland. Histochemical and electron microscopic evidence for their amyloid nature. J Comp Pathol 82:409–413
Rekas A, Adda CG, Andrew Aquilina J, Barnham KJ, Sunde M, Galatis D, Williamson NA, Masters CL, Anders RF, Robinson CV, Cappai R, Carver JA (2004) Interaction of the molecular chaperone alphaB-crystallin with alpha-synuclein: effects on amyloid fibril formation and chaperone activity. J Mol Biol 340:1167–1183
Rekas A, Jankova L, Thorn DC, Cappai R, Carver JA (2007) Monitoring the prevention of amyloid fibril formation by alpha-crystallin. Temperature dependence and the nature of the aggregating species. FEBS J 274:6290–6304
Renkawek K, Voorter CE, Bosman GJ, van Workum FP, de Jong WW (1994) Expression of alpha B-crystallin in Alzheimer's disease. Acta Neuropathol 87:155–160
Robertson AL, Headey SJ, Saunders HM, Ecroyd H, Scanlon MJ, Carver JA, Bottomley SP (2010) Small heat-shock proteins inhibit polyglutamine aggregation by interactions with a flanking domain. Proc Natl Acad Sci USA 107:10424–10429
Roychaudhuri R, Yang M, Hoshi MM, Teplow DB (2009) Amyloid beta-protein assembly and Alzheimer disease. J Biol Chem 284:4749–4753
Santhoshkumar P, Sharma KK (2004) Inhibition of amyloid fibrillogenesis and toxicity by a peptide chaperone. Mol Cell Biochem 267:147–155
Shinohara H, Inaguma Y, Goto S, Inagaki T, Kato K (1993) Alpha B crystallin and HSP28 are enhanced in the cerebral cortex of patients with Alzheimer's disease. J Neurol Sci 119:203–208
Simoneau S, Rezaei H, Sales N, Kaiser-Schulz G, Lefebvre-Roque M, Vidal C, Fournier JG, Comte J, Wopfner F, Grosclaude J, Schatzl H, Lasmezas CI (2007) In vitro and in vivo neurotoxicity of prion protein oligomers. PLoS Pathog 3:e125
Stefani M, Dobson CM (2003) Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med 81:678–699
Stege GJ, Renkawek K, Overkamp PS, Verschuure P, van Rijk AF, Reijnen-Aalbers A, Boelens WC, Bosman GJ, de Jong WW (1999) The molecular chaperone alphaB-crystallin enhances amyloid beta neurotoxicity. Biochem Biophys Res Commun 262:152–156
Tanaka Y, Engelender S, Igarashi S, Rao RK, Wanner T, Tanzi RE, Sawa A, LD V, Dawson TM, Ross CA (2001) Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum Mol Genet 10:919–926
Taniyama H, Kitamura A, Kagawa Y, Hirayama K, Yoshino T, Kamiya S (2000) Localized amyloidosis in canine mammary tumors. Vet Pathol 37:104–107
Thorn DC, Meehan S, Sunde M, Rekas A, Gras SL, MacPhee CE, Dobson CM, Wilson MR, Carver JA (2005) Amyloid fibril formation by bovine milk kappa-casein and its inhibition by the molecular chaperones alphaS- and beta-casein. Biochemistry 44:17027–17036
Thorn DC, Ecroyd H, Sunde M, Poon S, Carver JA (2008) Amyloid fibril formation by bovine milk alpha s2-casein occurs under physiological conditions yet is prevented by its natural counterpart, alpha s1-casein. Biochemistry 47:3926–3936
Thorn DC, Ecroyd H, Carver JA (2009) The two-faced nature of the milk casein proteins: amyloid fibril formation and chaperone-like activity. Aust J Dairy Technol 64:26–32
Troy CM, Rabacchi SA, Friedman WJ, Frappier TF, Brown K, Shelanski ML (2000) Caspase-2 mediates neuronal cell death induced by beta-amyloid. J Neurosci 20:1386–1392
Troy CM, Rabacchi SA, Xu Z, Maroney AC, Connors TJ, Shelanski ML, Greene LA (2001) beta-Amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem 77:157–164
Vreeman HJ, Brinkhuis JA, van der Spek CA (1981) Some association properties of bovine SH-kappa-casein. Biophys Chem 14:185–193
Ward RV, Jennings KH, Jepras R, Neville W, Owen DE, Hawkins J, Christie G, Davis JB, George A, Karran EH, Howlett DR (2000) Fractionation and characterization of oligomeric, protofibrillar and fibrillar forms of beta-amyloid peptide. Biochem J 348:137–144
Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, de Waal RM, Verbeek MM (2006a) Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain Res 1089:67–78
Wilhelmus MM, Otte-Holler I, Wesseling P, de Waal RM, Boelens WC, Verbeek MM (2006b) Specific association of small heat shock proteins with the pathological hallmarks of Alzheimer's disease brains. Neuropathol Appl Neurobiol 32:119–130
Yankner BA, Lu T (2009) Amyloid beta-protein toxicity and the pathogenesis of Alzheimer disease. J Biol Chem 284:4755–4759
Acknowledgments
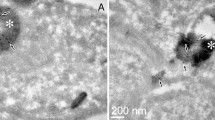
We thank Dr. Lyn Waterhouse (Medical School, University of Adelaide) for assistance with the TEM and David Thorn for helpful conversations. This work was supported by a grant from the Australian Research Council to J.A.C. H.E. was supported by a National Health and Medical Research Council Peter Doherty Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dehle, F.C., Ecroyd, H., Musgrave, I.F. et al. αB-Crystallin inhibits the cell toxicity associated with amyloid fibril formation by κ-casein and the amyloid-β peptide. Cell Stress and Chaperones 15, 1013–1026 (2010). https://doi.org/10.1007/s12192-010-0212-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-010-0212-z