Abstract
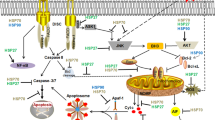
The cellular response to heat shock (HS) is a paradigm for many human diseases collectively known as “protein conformation diseases” in which the accumulation of misfolded proteins induces cell death. Here, we analyzed how cells having a different apoptotic threshold die subsequent to a treatment with HS. Cells with a low apoptotic threshold mainly induced apoptosis through activation of conventional stress kinase signaling pathways. By contrast, cells with a high apoptotic threshold also died by apoptosis but likely after the accumulation of heat-aggregated proteins as revealed by the formation of aggresomes in these cells, which were associated with the generation of atypical nuclear deformations. Inhibition of the proteasome or expression of an aggregation prone protein produced similar nuclear alterations. Furthermore, elevated levels of chaperones markedly suppressed both HS-induced nuclear deformations and apoptosis induced upon protein aggregation whereas they had little effect on stress kinase-mediated apoptosis. We conclude that the relative contribution of stress signaling pathways and the accumulation of protein aggregates to cell death by apoptosis is related to the innate sensitivity of cells to deadly insults.









Similar content being viewed by others
References
Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM (2002) Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295:865–868
Bailey CK, Andriola IF, Kampinga HH, Merry DE (2002) Molecular chaperones enhance the degradation of expanded polyglutamine repeat androgen receptor in a cellular model of spinal and bulbar muscular atrophy. Hum Mol Genet 11:515–523
Bennett EJ, Bence NF, Jayakumar R, Kopito RR (2005) Global impairment of the ubiquitin–proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol Cell 17:351–365
Bredesen DE, Rao RV, Mehlen P (2006) Cell death in the nervous system. Nature 443:796–802
Broers JL, Bronnenberg NM, Kuijpers HJ, Schutte B, Hutchison CJ, Ramaekers FC (2002) Partial cleavage of A-type lamins concurs with their total disintegration from the nuclear lamina during apoptosis. Eur J Cell Biol 81:677–691
Bucciantini M, Giannoni E, Chiti F et al (2002) Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416:507–511
Cao X, Bennett RL, May WS (2008) c-Myc and caspase-2 are involved in activating Bax during cytotoxic drug-induced apoptosis. J Biol Chem 283:14490–14496
Chang HY, Nishitoh H, Yang X, Ichijo H, Baltimore D (1998) Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 281:1860–1863
Charette SJ, Lavoie JN, Lambert H, Landry J (2000) Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol 20:7602–7612
Cummings CJ, Sun Y, Opal P et al (2001) Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet 10:1511–1518
Desbiens KM, Deschesnes RG, Labrie MM, Desfosses Y, Lambert H, Landry J, Bellmann K (2003) c-Myc potentiates the mitochondrial pathway of apoptosis by acting upstream of apoptosis signal-regulating kinase 1 (Ask1) in the p38 signalling cascade. Biochem J 372:631–641
Evan GI, Wyllie AH, Gilbert CS et al (1992) Induction of apoptosis in fibroblasts by c-myc protein. Cell 69:119–128
Fortun J, Dunn WA Jr, Joy S, Li J, Notterpek L (2003) Emerging role for autophagy in the removal of aggresomes in Schwann cells. J Neurosci 23:10672–10680
Friant S, Meier KD, Riezman H (2003) Increased ubiquitin-dependent degradation can replace the essential requirement for heat shock protein induction. EMBO J 22:3783–3791
Gabai VL, Yaglom JA, Volloch V et al (2000) Hsp72-mediated suppression of c-Jun N-terminal kinase is implicated in development of tolerance to caspase-independent cell death. Mol Cell Biol 20:6826–6836
Glover JR, Lindquist S (1998) Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94:73–82
Hohfeld J, Cyr DM, Patterson C (2001) From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep 2:885–890
Huot J, Houle F, Spitz DR, Landry J (1996) HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res 56:273–279
Hut HM, Kampinga HH, Sibon OC (2005) Hsp70 protects mitotic cells against heat-induced centrosome damage and division abnormalities. Mol Biol Cell 16:3776–3785
Johnston JA, Ward CL, Kopito RR (1998) Aggresomes: a cellular response to misfolded proteins. J Cell Biol 143:1883–1898
Juin P, Hunt A, Littlewood T, Griffiths B, Swigart LB, Korsmeyer S, Evan G (2002) c-Myc functionally cooperates with Bax to induce apoptosis. Mol Cell Biol 22:6158–6169
Kopito RR (2000) Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol 10:524–530
Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92:2177–2186
Landry J, Chretien P, Lambert H, Hickey E, Weber LA (1989) Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol 109:7–15
Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55:1151–1191
Littlewood TD, Hancock DC, Danielian PS, Parker MG, Evan GI (1995) A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res 23:1686–1690
Maulik N, Das DK (2008) Emerging potential of thioredoxin and thioredoxin interacting proteins in various disease conditions. Biochim Biophys Acta 1780:1368–1382
Michels AA, Kanon B, Konings AW, Bensaude O, Kampinga HH (2000) Cycloheximide- and puromycin-induced heat resistance: different effects on cytoplasmic and nuclear luciferases. Cell Stress Chaperones 5:181–187
Moir RD, Yoon M, Khuon S, Goldman RD (2000) Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J Cell Biol 151:1155–1168
Nadeau SI, Landry J (2007) Mechanisms of activation and regulation of the heat shock-sensitive signaling pathways. Adv Exp Med Biol 594:100–113
Perche PY, Vourc'h C, Konecny L, Souchier C, Robert-Nicoud M, Dimitrov S, Khochbin S (2000) Higher concentrations of histone macroH2A in the Barr body are correlated with higher nucleosome density. Curr Biol 10:1531–1534
Salomons FA, Menendez-Benito V, Bottcher C, McCray BA, Taylor JP, Dantuma NP (2009) Selective accumulation of aggregation-prone proteasome substrates in response to proteotoxic stress. Mol Cell Biol 29:1774–1785
Vidair CA, Doxsey SJ, Dewey WC (1993) Heat shock alters centrosome organization leading to mitotic dysfunction and cell death. J Cell Physiol 154:443–455
Waelter S, Boeddrich A, Lurz R, Scherzinger E, Lueder G, Lehrach H, Wanker EE (2001) Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol Biol Cell 12:1393–1407
Ward CL, Omura S, Kopito RR (1995) Degradation of CFTR by the ubiquitin–proteasome pathway. Cell 83:121–127
Wigley WC, Fabunmi RP, Lee MG, Marino CR, Muallem S, DeMartino GN, Thomas PJ (1999) Dynamic association of proteasomal machinery with the centrosome. J Cell Biol 145:481–490
Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 139:1281–1292
Wyttenbach A, Carmichael J, Swartz J, Furlong RA, Narain Y, Rankin J, Rubinsztein DC (2000) Effects of heat shock, heat shock protein 40 (HDJ-2), and proteasome inhibition on protein aggregation in cellular models of Huntington's disease. Proc Natl Acad Sci U S A 97:2898–2903
Acknowledgments
We thank R.J. Youle, D. Baltimore, R.D. Goldman, P.Y. Perche, D.C. Rubinsztein, and R.R. Kopito for their generous contribution of reagents. This study was supported by the Canadian Institutes of Health Research Grant MOP-7088 and the Canada Research Chair in Stress Signal Transduction.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kerstin Bellmann and Steve J. Charette have equal contribution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Typical examples of cell fate obtained by live cell imaging. Rat1-control cells were transfected with H2A-GFP and then heated for 45 min at 43.5°C (B) or not (A) before their analysis by live imaging. Each horizontal line represents one living cell. Small vertical lines indicate mitotic events. Periods of time where cells displayed a typical HS-induced nuclear deformation are illustrated by dotted lines. Asterisks indicate apoptotic events. Numbers indicate time after HS in hour
Movie of Rat1-control cells expressing H2A-GFP after HS treatment. Rat1-control cells were transfected with H2A-GFP and then heated for 45 min at 43.5°C before their analysis by live imaging. The movie starts 1 h after HS treatment and covers a period of 24 h post-HS (one image every 30 min). All the nuclei present a deformed morphology. Two apoptotic events occur (one in the middle of the field and a second in the top right of the field after a cell division). (MOV 2355 kb)
Movie of Rat1-myc cells expressing H2A-GFP after HS treatment. Rat1-myc cells were transfected with H2A-GFP and then heated for 45 min at 43.5°C before their analysis by live imaging. The movie starts 1 h after HS treatment and covers a period of 24 h post-HS (one image every 30 min). All the nuclei present a deformed morphology and then the cells die by apoptosis. (MOV 1197 kb)
Rights and permissions
About this article
Cite this article
Bellmann, K., Charette, S.J., Nadeau, P.J. et al. The mechanism whereby heat shock induces apoptosis depends on the innate sensitivity of cells to stress. Cell Stress and Chaperones 15, 101–113 (2010). https://doi.org/10.1007/s12192-009-0126-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-009-0126-9