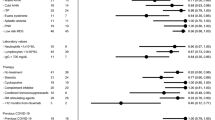
Abstract
Aplastic anemia (AA) is a rare autoimmune disease. Drugs, viruses, and radiation are among the most common etiologic factors, and most cases have immune pathophysiology. SARS-CoV-2 vaccines have been linked with rare side effects, including cases of acquired aplastic anemia. Here we review all the reported cases of new-onset AA after SARS-CoV-2 vaccination, and discuss their clinical characteristics and management. 18 patients in these case reports had a median age of 58 years. The time from vaccination to onset of aplastic anemia ranged from 1 day to 7 months, with a median of 2.5 weeks. Seventeen patients were diagnosed with severe or very severe aplastic anemia post-vaccination and all patients received standard treatments for acquired aplastic anemia. Seventeen patients achieved a complete or partial response and only 1 patient died. Aplastic anemia can be considered a very rare SARS-CoV-2 vaccine-related adverse event, although a causative relationship has not been proven. Reporting cases of such uncommon post-vaccination events could help clinicians to consider aplastic anemia when pancytopenia is observed after vaccination. The benefits of SARS-Cov-2 vaccination are established, and reports of rare events serve only to increase awareness in daily clinical practice.
Similar content being viewed by others
Abbreviations
- AA:
-
Aplastic anemia
- ACST:
-
Allogeneic stem cell transplantation
- AE:
-
Adverse event
- ANC:
-
Absolute neutrophil count
- ATG:
-
Anti-thymocyte globulin
- BM:
-
Bone marrow
- Hb:
-
Hemoglobin
- HLA:
-
Human leukocyte antigen
- IST:
-
Immunosuppressive therapy
- PLT:
-
Platelets
- PNH:
-
Paroxysmal nocturnal Hemoglobinuria
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SCT:
-
Stem cell transplantation
References
Giudice V, Selleri C. Aplastic anemia: pathophysiology. Semin Hematol. 2022;59(1):13–20. https://doi.org/10.1053/j.seminhematol.2021.12.002. (Epub 2022 Jan 5 PMID: 35491054).
Solomou EE, Gibellini F, Stewart B, et al. Perforin gene mutations in patients with acquired aplastic anemia. Blood. 2007;109(12):5234–7. https://doi.org/10.1182/blood-2006-12-063495.
Young NS. Acquired aplastic anemia. Ann Intern Med. 2002;136(7):534–46. https://doi.org/10.7326/0003-4819-136-7-200204020-00011. (PMID: 11926789).
Viallard JF, Boiron JM, Parrens M, Moreau JF, Ranchin V, Reiffers J, Leng B, Pellegrin JL. Severe pancytopenia triggered by recombinant hepatitis B vaccine. Br J Haematol. 2000;110(1):230–3. https://doi.org/10.1046/j.1365-2141.2000.02171.x. (PMID: 10931005).
Hendry CL, Sivakumaran M, Marsh JC, Gordon-Smith EC. Relapse of severe aplastic anaemia after influenza immunization. Br J Haematol. 2002;119(1):283–4. https://doi.org/10.1046/j.1365-2141.2002.379111.x. (PMID: 12358945).
Angelini P, Kavadas F, Sharma N, Richardson SE, Tipples G, Roifman C, Dror Y, Nofech-Mozes Y. Aplastic anemia following varicella vaccine. Pediatr Infect Dis J. 2009;28(8):746–8. https://doi.org/10.1097/INF.0b013e31819b6c1f. (PMID: 19633522).
Ritz C, Meng W, Stanley NL, Baroja ML, Xu C, Yan P, Huang AC, Hausler R, Nicholas P, Fan JM, Lieberman D, Carreno BM, Luning Prak ET, Olson TS, Babushok DV. Postvaccination graft dysfunction/aplastic anemia relapse with massive clonal expansion of autologous CD8+ lymphocytes. Blood Adv. 2020;4(7):1378–82. https://doi.org/10.1182/bloodadvances.2019000853.Erratum.In:BloodAdv.2020Jul14;4(13):2865.PMID:32267929;PMCID:PMC7160279.
Al-Ali D, Elshafeey A, Mushannen M, Kawas H, Shafiq A, Mhaimeed N, Mhaimeed O, Mhaimeed N, Zeghlache R, Salameh M, Paul P, Homssi M, Mohammed I, Narangoli A, Yagan L, Khanjar B, Laws S, Elshazly MB, Zakaria D. Cardiovascular and haematological events post COVID-19 vaccination: a systematic review. J Cell Mol Med. 2022;26(3):636–53. https://doi.org/10.1111/jcmm.17137. (Epub 2021 Dec 29. PMID: 34967105; PMCID: PMC8817142).
Fatima Z, Reece BRA, Moore JS, Means RT Jr. Autoimmune Hemolytic Anemia After mRNA COVID Vaccine. J Investig Med High Impact Case Rep. 2022;10:23247096211073256. https://doi.org/10.1177/23247096211073258. (PMID: 35045762; PMCID: PMC8777324).
Herblum J, Frishman W. Cardiovascular and Hematologic Complications of COVID-19 Vaccines. Cardiol Rev. 2022. https://doi.org/10.1097/CRD.0000000000000457. (Epub ahead of print. PMID: 35576367).
Allen JD, Feng W, Corlin L, Porteny T, Acevedo A, Schildkraut D, King E, Ladin K, Fu Q, Stopka TJ. Why are some people reluctant to be vaccinated for COVID-19? A cross-sectional survey among U.S. Adults in May–June 2020. Prev Med Rep. 2021;24:101494. https://doi.org/10.1016/j.pmedr.2021.101494. (Epub 2021 Jul 14. PMID: 34277329; PMCID: PMC8277541).
Graña C, Ghosn L, Evrenoglou T, Jarde A, Minozzi S, Bergman H, Buckley BS, Probyn K, Villanueva G, Henschke N, Bonnet H, Assi R, Menon S, Marti M, Devane D, Mallon P, Lelievre JD, Askie LM, Kredo T, Ferrand G, Davidson M, Riveros C, Tovey D, Meerpohl JJ, Grasselli G, Rada G, Hróbjartsson A, Ravaud P, Chaimani A, Boutron I. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev. 2022;12(12):CD015477. https://doi.org/10.1002/14651858.CD015477. (PMID: 36473651; PMCID: PMC9726273).
Anand P, Stahel VP. Review the safety of Covid-19 mRNA vaccines: a review. Patient Saf Surg. 2021;15(1):20. https://doi.org/10.1186/s13037-021-00291-9.Erratum.In:PatientSafSurg.2021May18;15(1):22.PMID:33933145;PMCID:PMC8087878.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU. Gruber WC C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–15. https://doi.org/10.1056/NEJMoa2034577. (Epub 2020 Dec 10. PMID: 33301246; PMCID: PMC7745181).
Al Khames Aga QA, Alkhaffaf WH, Hatem TH, Nassir KF, Batineh Y, Dahham AT, Shaban D, Al Khames Aga LA, Agha MYR, Traqchi M. Safety of COVID-19 vaccines. J Med Virol. 2021;93(12):6588–94. https://doi.org/10.1002/jmv.27214. (Epub 2021 Jul 28. PMID: 34270094; PMCID: PMC8426829).
Singh A, Khillan R, Mishra Y, Khurana S. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50(1):15–9. https://doi.org/10.1016/j.ajic.2021.10.015. (Epub 2021 Oct 23. PMID: 34699960; PMCID: PMC8539830).
Cecchi N, Giannotta JA, Barcellini W, Fattizzo B. A case of severe aplastic anaemia after SARS-CoV-2 vaccination. Br J Haematol. 2022;196(6):1334–6.
Chen CY, Chen TT, Hsieh CY, et al. Case reports of management of aplastic anemia after COVID-19 vaccination: a single institute experience in Taiwan. Int J Hematol. 2023;117(1):149–52.
Babakhanlou R, Kadia T, Chien K, Thompson P. P807: aplastic anemia following the sars-cov-2 vaccine. HemaSphere. 2022;6:701–2. https://doi.org/10.1097/01.HS9.0000846112.16677.4.
Ross Lavine, MD, Anna-Belle Robertson, MBBS, Jason Lofters, MBBS, Jalisa Carvalho, MD, Anesha White, MD, Michael Basir, MBBS, Hassan Alkhatatneh, MD, Jodi-Ann Smith, MBBS, Alexandra Gottdiener, MD. Severe aplastic anemia post covid-19 vaccination in young male found to be a carrier of fanconi anemia type C. Abstract published at SHM Converge 2022. Abstract D29, Journal of Hospital Medicine. https://shmabstracts.org/abstract/severe-aplastic-anemia-post-covid-19-vaccination-in-young-male-found-to-be-a-carrier-of-fanconi-anemia-type-c/. September 26th 2022.
Tabata S, Hosoi H, Murata S, Takeda S, Mushino T, Sonoki T. Severe aplastic anemia after COVID-19 mRNA vaccination: Causality or coincidence? J Autoimmun. 2022;126:102782. https://doi.org/10.1016/j.jaut.2021.102782.
Sridhara S, Nair R, Stanek M. Severe aplastic anemia after receiving SARS-CoV-2 moderna mRNA vaccination. J Hematol. 2022;11(1):34–9. https://doi.org/10.14740/jh954.
Yang X, Laczko D, Caponetti GC, Rabatin S, Babushok DV Severe aplastic anaemia after serial vaccinations for SARS-CoV-2, pneumococcus and seasonal influenza, eJHaem 2022. 10.1002/ jha2.443.
Woo S, Kim B, Lee SC, Kim MS, Yoon YA, Choi YJ. Very severe immune aplastic anemia after mRNA vaccination against COVID-19 responds well to immunosuppressive therapy: clinical characteristics and comparison to previous reports. Hematology. 2022;27(1):1191–5. https://doi.org/10.1080/16078454.2022.2140986.
Röth A, Bertram S, Schroeder T, Haverkamp T, Voigt S, Holtkamp C, Klump H, Wörmann B, Reinhardt HC, Alashkar F. Acquired aplastic anemia following SARS-CoV-2 vaccination. Eur J Haematol. 2022;109(2):186–94. https://doi.org/10.1111/ejh.13788.
Yamamoto M, Keino D, Sumii S, Yokosuka T, Goto H, Inui A, Sogo T, Kawakami M, Tanaka M, Yanagimachi M. Severe hepatitis-associated aplastic anemia following COVID-19 mRNA vaccination. Int Med. 2023;62(12):1813–6.
Kobayashi M, Mori A, Oda Y, et al. New onset of hypomegakaryocytic thrombocytopenia with the potential for progression to aplastic anemia after BNT162b2 mRNA COVID-19 vaccination. Int J Hematol. 2023. https://doi.org/10.1007/s12185-023-03618-7.
Peslak SA, Olson T, Babushok DV. Diagnosis and treatment of aplastic anemia. Curr Treat Options Oncol. 2017;18(12):70. https://doi.org/10.1007/s11864-017-0511-z.PMID:29143887;PMCID:PMC5804354.
Soriano A, Nesher G, Shoenfeld Y. Predicting post-vaccination autoimmunity: Who might be at risk? Pharmacol Res. 2015;92:18–22.
Shah C, Lemke S, Singh V, Gentile T. Case reports of aplastic anemia after vaccine administration. Am J Hematol. 2004;77(2):204. https://doi.org/10.1002/ajh.20153. (PMID: 15389916).
Donnini I, Scappini B, Guidi S, Longo G, Bosi A. Acquired severe aplastic anemia after H1N1 influenza virus vaccination successfully treated with allogeneic bone marrow transplantation. Ann Hematol. 2012;91(3):475–6. https://doi.org/10.1007/s00277-011-1278-0. (Epub 2011 Jun 17 PMID: 21681390).
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Marc GP, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–15.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert MD, Spector SA, Rouphael N, Creech B, et al. Efficacy and Safety of the mRNA-1273SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–16.
Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–78.
Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–201.
Avenoso D, Marsh JCW, Potter V, Pagliuca A, Slade S, Dignan F, Tholouli E, Mittal S, Davis B, Tauro S, Kesse-Adu R, Griffin M, Payne E, Gandhi S, Kulasekararaj AG. SARS-CoV-2 infection in aplastic anemia. Haematologica. 2022;107(2):541–3. https://doi.org/10.3324/haematol.2021.279928.PMID:34670361;PMCID:PMC8804560.
Barcellini W, Giannotta JA, Fattizzo B. Are patients with autoimmune cytopenias at higher risk of COVID-19 pneumonia? The experience of a reference center in northern italy and review of the literature. Front Immunol. 2021;11:609198. https://doi.org/10.3389/fimmu.2020.609198. (PMID: 33574816; PMCID: PMC7870679).
Fattizzo B, Giannotta JA, Cecchi N, Barcellini W. SARS-CoV-2 vaccination in patients with autoimmune cytopenias: The experience of a reference center. Am J Hematol. 2021;96(11):E413–6. https://doi.org/10.1002/ajh.26345. (Epub 2021 Sep 16. PMID: 34478178; PMCID: PMC8646755).
Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, Tobar A, Shoenfeld Y. Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. J Clin Invest. 2002;109(6):797–804. https://doi.org/10.1172/JCI12337.PMID:11901188;PMCID:PMC150905.
Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al., editors. Bogota (Colombia) El Rosario University Press; Chapter 21 Molecular mimicry in autoimmunity and vaccinations, Miri Blank, Eitan Israeli, Smadar Gertel, Howard Amital, and Yehuda Shoenfeld, National library of medicine, Autoimmunity: From Bench to Bedside, 2013, Jul 18 .
Amital H, Gershwin ME, Shoenfeld Y. Reshaping the mosaic of autoimmunity. Semin Arthritis Rheum. 2006;35(6):341–3. https://doi.org/10.1016/j.semarthrit.2005.09.002. (PMID: 16765709).
Barnett LA, Fujinami RS. Molecular mimicry: a mechanism for autoimmune injury. FASEB J. 1992;6(3):840–4. https://doi.org/10.1096/fasebj.6.3.1740233. (PMID: 1740233).
Farris AD, Keech CL, Gordon TP, McCluskey J. Epitope mimics and determinant spreading: pathways to autoimmunity. Cell Mol Life Sci. 2000;57(4):569–78. https://doi.org/10.1007/PL00000719. (PMID: 11130457).
Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19(1):80–94. https://doi.org/10.1128/CMR.19.1.80-94.2006.PMID:16418524;PMCID:PMC1360274.
Ebringer A, Rashid T, Wilson C. Rheumatoid arthritis, proteus, anti-CCP antibodies and Karl Popper. Autoimmun Rev. 2010;9(4):216–23. https://doi.org/10.1016/j.autrev.2009.10.006. (Epub 2009 Nov 4 PMID: 19895906).
Shoenfeld Y, Agmon-Levin N. ’ASIA’—autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36(1):4–8. https://doi.org/10.1016/j.jaut.2010.07.003. (Epub 2010 Aug 13 PMID: 20708902).
Taefehshokr N, Taefehshokr S, Heit B. Mechanisms of dysregulated humoral and cellular immunity by SARS-CoV-2. Pathogens. 2020;9(12):1027. https://doi.org/10.3390/pathogens9121027.PMID:33302366;PMCID:PMC7762606.
Bunders MJ, Altfeld M. Implications of sex differences in immunity for SARS-CoV-2 pathogenesis and design of therapeutic interventions. Immunity. 2020;53(3):487–95.
Lyden DC et al. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol 126.3 (1987): 432.
Lagousi T, Papadatou I, Strempas P, Chatzikalil E, Spoulou V. Paving the way towards precision vaccinology: the paradigm of myocarditis after coronavirus disease 2019 (COVID-19) vaccination. Clin Infect Dis. 2022;75(Suppl 1):S18–23.
Vaht K, Göransson M, Carlson K, Isaksson C, Lenhoff S, Sandstedt A, Uggla B, Winiarski J, Ljungman P, Brune M, Andersson PO. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102(10):1683–90. https://doi.org/10.3324/haematol.2017.169862. (Epub 2017 Jul 27. PMID: 28751565; PMCID: PMC5622852).
Solomou EE. Idiopathic aplastic anemia: an update. Clin Hematol Int. 2019;1(1):52–7. https://doi.org/10.2991/chi.d.190321.002.PMID:34595411;PMCID:PMC8432379.
Francesca Di Rosa, Thomas Gebhardt Pathogenesis of acquired aplastic anemia and the role of the bone marrow microenvironment. Front Oncol 05 December 2018, Sec. CancerImmunityandImmunotherapy 2018; 8 10.3389/ fonc.2018.00587
Zaimoku Y, Patel BA, Shalhoub R, Groarke EM, Feng X, Wu CO, Young NS. Predicting response of severe aplastic anemia to immunosuppression combined with eltrombopag. Haematologica. 2022;107(1):126–33. https://doi.org/10.3324/haematol.2021.278413.PMID:33910334;PMCID:PMC8719075.
Sánchez-Saez F, Peiró S, Cuenca L, Vanaclocha H, Limón R, Salas D, Burgos JS, Sánchez-Payá J, Meneu R, Díez J, García-Sempere A, Navarro IH, Rodríguez-Bernal C, Sanfélix-Gimeno G, Navarro D. Valencian vaccine research program (ProVaVac) study group Side effects during the week after first dose vaccination with four Covid-19 vaccines Results of the ProVaVac Survey Study with 13,837 people in Spain. Vaccine. 2022;40(41):5942–9. https://doi.org/10.1016/j.vaccine.2022.08.028. (Epub 2022 Aug 29. PMID: 36068110; PMCID: PMC9420721).
Yamamoto K. Adverse effects of COVID-19 vaccines and measures to prevent them. Virol J. 2022;19(1):100. https://doi.org/10.1186/s12985-022-01831-0.PMID:35659687;PMCID:PMC9167431.
Nordström P, Ballin M, Nordström A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden. Lancet. 2022;399(10327):814–23. https://doi.org/10.1016/S0140-6736(22)00089-7.
Seneff S, Nigh G, Kyriakopoulos AM, McCullough PA. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: the role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem Toxicol. 2022;164:113008. https://doi.org/10.1016/j.fct.2022.113008.
Stone CA Jr, Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85(12):2694–706. https://doi.org/10.1111/bcp.14112. (Epub 2019 Nov 5. PMID: 31472022; PMCID: PMC6955412).
Pozzetto B, Legros V, Djebali S, Barateau V, Guibert N, Villard M, Peyrot L, Allatif O, Fassier JB, Massardier-Pilonchéry A, Brengel-Pesce K. Immunogenicity and efficacy of heterologous ChAdOx1–BNT162b2 vaccination. Nature. 2021;600(7890):701–6.
Nham E, Kim YE, Jung J, Kim DW, Jang H, Hyun H, Seong H, Yoon JG, Noh JY, Song JY, Kim WJ, Cheong HJ. COVID-19 vaccination rates in patients with chronic medical conditions: a nationwide cross-sectional study. J Korean Med Sci. 2022;37(45):e325. https://doi.org/10.3346/jkms.2022.37.e325. (PMID: 36413798; PMCID: PMC9678655).
Pal R, Bhadada SK, Misra A. COVID-19 vaccination in patients with diabetes mellitus: Current concepts, uncertainties and challenges. Diabetes Metab Syndr. 2021;15(2):505–8. https://doi.org/10.1016/j.dsx.2021.02.026. (Epub 2021 Feb 25. PMID: 33662837; PMCID: PMC7904463).
Xiong X, Wong CKH, Au ICH, Lai FTT, Li X, Wan EYF, Chui CSL, Chan EWY, Cheng FWT, Lau KTK, Lee CH, Woo YC, Lui DTW, Wong ICK. Safety of inactivated and mRNA COVID-19 vaccination among patients treated for hypothyroidism: a population-based cohort study. Thyroid. 2022;32(5):505–14. https://doi.org/10.1089/thy.2021.0684. (Epub 2022 Apr 7 PMID: 35216517).
Immune response and Adverse Events after vaccination against SARS-COVID-19 Vaccination in adult patients with Transfusion-Dependent -Thalassemia Patients, Delaporta, P. Terpos E, Solomou EE, Gumeni S, Nitsa E, Apostolakou F Kyriakopoulou, D.; Stathopoulos I, Papassotiriou, I. Trougakos I, Dimopoulos M, Kattamis, A.., Br J Haematol ; 2022; 197: 576.
Baldolli A, Michon J, Appia F, Galimard C, Verdon R, Parienti JJ. Tolerance of BNT162b2 mRNA COVI-19 vaccine in patients with a medical history of COVID-19 disease: a case control study. Vaccine. 2021;39(32):4410–3. https://doi.org/10.1016/j.vaccine.2021.06.054. (Epub 2021 Jun 23. PMID: 34210574; PMCID: PMC8220908).
Fahmy O, Ayad A, Marie M, Saber ES, Hamza MT, El Demerdash D Post COVID 19 aplastic anemia: case report, HemaSphere ; 5(SUPPL 2):706, 2021, Artigo em Inglês | EMBASE | ID: covidwho-1393393.
Sumbly V, Siddiqui R, Alshamam M, Kurbanova T, Rizzo V. New onset aplastic anemia after a COVID-19 infection: a case report. Am J Med Case Rep. 2021;9:451–5.
Lee NCJ, Patel B, Etra A, Bat T, Ibrahim IF, Vusirikala M, Chen M, Rosado F, Jaso JM, Young NS, Chen W. SARS-CoV-2 infection associated with aplastic anemia and pure red cell aplasia. Blood Adv. 2022;6(13):3840–3. https://doi.org/10.1182/bloodadvances.2022007174.PMID:35452511;PMCID:PMC9040401.
Pascutti MF, Erkelens MN, Nolte MA. Impact of viral infections on hematopoiesis: from beneficial to detrimental effects on bone marrow output. Front Immunol. 2016;16(7):364. https://doi.org/10.3389/fimmu.2016.00364.PMID:27695457;PMCID:PMC5025449.
Lagadinou M, Solomou EE, Zareifopoulos N, Marangos M, Gogos C, Velissaris D. Infez Med. 2020;28(Suppl 1):89–95.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Chatzikalil, E., Kattamis, A., Diamantopoulos, P. et al. New-onset aplastic anemia after SARS-CoV-2 vaccination. Int J Hematol 118, 667–681 (2023). https://doi.org/10.1007/s12185-023-03666-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-023-03666-z