Abstract
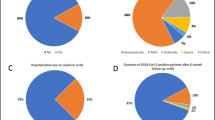
Pre-exposure prophylaxis with tixagevimab/cilgavimab was considered a useful strategy to protect immunocompromised patients from COVID-19 based on the phase 3 PROVENT trial conducted between November 2020 and March 2021. However, after late 2021, the dominant substrains of COVID-19 changed to Omicron substrains, which showed resistance to tixagevimab/cilgavimab. Therefore, it is important to re-evaluate the real-world efficacy of tixagevimab/cilgavimab for the prevention of COVID-19 in the Omicron era. To this end, we retrospectively evaluated the efficacy and safety of tixagevimab/cilgavimab prophylaxis for COVID-19 during the Omicron BA.5 wave in Japan. A total of 240 consecutive patients with hematologic malignancies received tixagevimab/cilgavimab at our institution from October 18, 2022, to January 31, 2023. Among them, the cumulative incidence of COVID-19 at 90 days was 6.4%. A total of 10/14 (71.4%) had mild infection, and 4/14 (28.5%) had severe infection. No patient died due to COVID-19. Adverse events consisted of deep vein thrombosis in 2 patients. Our analysis indicated that pre-exposure prophylaxis with tixagevimab/cilgavimab might have clinical effectiveness in reducing the severity of COVID-19 in Japanese HM patients, even in the Omicron BA.5 surge. It also suggested that tixagevimab/cilgavimab may be associated with cardiovascular complications.

Similar content being viewed by others
Data availability
Data will be made available on request.
References
Lee LYW, Starkey T, Ionescu MC, Little M, Tilby M, Tripathy AR, et al. Vaccine effectiveness against COVID-19 breakthrough infections in patients with cancer (UKCCEP): a population-based test-negative case-control study. Lancet Oncol. 2022;23(6):748–57. https://doi.org/10.1016/S1470-2045(22)00202-9.
Pagano L, Salmanton-Garcia J, Marchesi F, Blennow O, Gomes da Silva M, Glenthoj A, et al. Breakthrough COVID-19 in vaccinated patients with hematologic malignancies: results from the EPICOVIDEHA survey. Blood. 2022;140(26):2773–87. https://doi.org/10.1182/blood.2022017257.
Langerbeins P, Hallek M. COVID-19 in patients with hematologic malignancy. Blood. 2022;140(3):236–52. https://doi.org/10.1182/blood.2021012251.
Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19. N Engl J Med. 2022;386(23):2188–200. https://doi.org/10.1056/NEJMoa2116620.
Kertes J, Shapiro Ben David S, Engel-Zohar N, Rosen K, Hemo B, Kantor A, et al. Association Between AZD7442 (Tixagevimab-Cilgavimab) Administration and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, Hospitalization, and Mortality. Clin Infect Dis. 2023;76(3):126–32. https://doi.org/10.1093/cid/ciac625.
Ocon AJ, Ocon KE, Battaglia J, Low SK, Neupane N, Saeed H, et al. Real-world effectiveness of tixagevimab and cilgavimab (Evusheld) in patients with hematological malignancies. J Hematol. 2022;11(6):210–5. https://doi.org/10.14740/jh1062.
Imai M, Ito M, Kiso M, Yamayoshi S, Uraki R, Fukushi S, et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. N Engl J Med. 2023;388(1):89–91. https://doi.org/10.1056/NEJMc2214302.
Davis JA, Granger K, Roubal K, Smith D, Gaffney KJ, McGann M, et al. Efficacy of tixagevimab-cilgavimab in preventing SARS-CoV-2 for patients with B-cell malignancies. Blood. 2023;141(2):200–3. https://doi.org/10.1182/blood.2022018283.
Chen B, Haste N, Binkin N, Law N, Horton LE, Yam N, et al. Real world effectiveness of tixagevimab/cilgavimab (Evusheld) in the Omicron era. PLoS ONE. 2023;18(4):e0275356. https://doi.org/10.1371/journal.pone.0275356.
Organization WH. Living guidance for clinical management of COVID-19. In: Organization WH, 2021.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. https://doi.org/10.1038/bmt.2012.244.
Jondreville L, D’Aveni M, Labussière-Wallet H, Le Bourgeois A, Villate A, Berceanu A, et al. Pre-exposure prophylaxis with tixagevimab/cilgavimab (AZD7442) prevents severe SARS-CoV-2 infection in recipients of allogeneic hematopoietic stem cell transplantation during the Omicron wave: a multicentric retrospective study of SFGM-TC. J Hematol Oncol. 2022;15(1):169. https://doi.org/10.1186/s13045-022-01387-0.
Mauro FR, Visentin A, Giannarelli D, Molinari MC, Proietti G, Petrella M, et al. Pre-exposure prophylaxis with tixagevimab/cilgavimab in patients with chronic lymphocytic leukaemia treated with targeted agents. Br J Haematol. 2023;201(3):564–7. https://doi.org/10.1111/bjh.18701.
Marchesi F, Salmanton-García J, Buquicchio C, Itri F, Besson C, Dávila-Valls J, et al. Passive pre-exposure immunization by tixagevimab/cilgavimab in patients with hematological malignancy and COVID-19: matched-paired analysis in the EPICOVIDEHA registry. J Hematol Oncol. 2023;16(1):32. https://doi.org/10.1186/s13045-023-01423-7.
Chang A, Koff JL, Lai L, Orellana-Noia VM, Surati M, Leal A, et al. Low neutralizing activity of AZD7442 against current SARS-CoV-2 Omicron variants in patients with B cell malignancies. Blood Adv. 2023. https://doi.org/10.1182/bloodadvances.2022009475.
Piszczek J, Murthy S, Afra K. Cardiac and vascular serious adverse events following tixagevimab-cilgavimab. Lancet Respir Med. 2023;11(1):e5–6. https://doi.org/10.1016/s2213-2600(22)00452-0.
Montastruc F, Lafaurie M, Flumian C, de Canecaude C. Increased reporting of venous and arterial thromboembolic events reported with tixagevimab-cilgavimab for coronavirus disease 2019. Clin Microbiol Infect. 2023;29(4):543.e1-543.e3. https://doi.org/10.1016/j.cmi.2022.11.026.
Maselkar S, Kiazand A, Templeton A, Montgomery H, Esser MT. Cardiac and vascular serious adverse events following tixagevimab-cilgavimab—Author’s reply. Lancet Respir Med. 2023;11(1):e7–8. https://doi.org/10.1016/S2213-2600(22)00450-7.
Birabaharan M, Hill E, Begur M, Kaelber DC, Martin TCS, Mehta SR. Cardiovascular outcomes after tixagevimab and cilgavimab use for pre-exposure prophylaxis against coronavirus disease 2019: a population-based propensity-matched Cohort Study. Clin Infect Dis. 2023;76(8):1500–3. https://doi.org/10.1093/cid/ciac894.
De Stefano V, Sora F, Rossi E, Chiusolo P, Laurenti L, Fianchi L, et al. The risk of thrombosis in patients with acute leukemia: occurrence of thrombosis at diagnosis and during treatment. J Thromb Haemost. 2005;3(9):1985–92. https://doi.org/10.1111/j.1538-7836.2005.01467.x.
Melody M, Gandhi S, Saunders H, Abdel-Rahman Z, Hastings J, Lengerke Diaz P, et al. Incidence of thrombosis in relapsed/refractory B-cell lymphoma treated with axicabtagene ciloleucel: Mayo Clinic experience. Leuk Lymphoma. 2022;63(6):1363–8. https://doi.org/10.1080/10428194.2022.2030475.
Terpos E, Musto P, Engelhardt M, Delforge M, Cook G, Gay F, et al. Management of patients with multiple myeloma and COVID-19 in the post pandemic era: a consensus paper from the European Myeloma Network (EMN). Leukemia. 2023. https://doi.org/10.1038/s41375-023-01920-1.
Acknowledgements
The authors are grateful to the contributions of all internal medicine outpatient nurses, seventh west floor nurses, and the Pharmaceutical Department at the University of Yamanashi Hospital.
Author information
Authors and Affiliations
Contributions
IK and KK designed the study, collected data, analyzed data, and wrote the manuscript. HH, AN, MM, YS, TK, MS, MK, TY, and MT performed patient care and collected data.
Corresponding author
Ethics declarations
Conflict of interest
KK received lecture fees from Takeda Pharmaceutical Company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kawashima, I., Hyuga, H., Nakadate, A. et al. Pre-exposure prophylaxis with tixagevimab/cilgavimab for coronavirus disease 2019 (COVID-19) during the Omicron BA.5 wave at a single institution in Japan. Int J Hematol 118, 731–736 (2023). https://doi.org/10.1007/s12185-023-03663-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-023-03663-2