Abstract
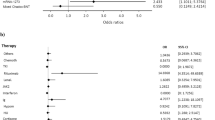
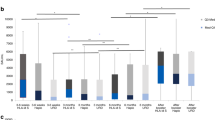
To determine the efficacy of SARS-CoV-2 mRNA vaccination for allogeneic hematopoietic stem cell transplantation (allo-HSCT) recipients, we measured antibody titer serially in 92 allo-HSCT patients. Among the evaluable 87 patients, median age at vaccination was 53 years (range, 18–75). The average time between allo-HSCT and vaccination was 3.3 years (range, 0.5–15.7). One month after the second dose, 70 patients (80.5%) had a positive response, whereas 17 patients (19.5%) had a negative response (< 20 U/mL). Only patients older than 44 years had a negative response. Low IgM level was the only significant predictor of vaccine failure in elderly patients. When antibody response before and after the third vaccination was examined in 47 patients, antibodies increased significantly from a median of 18.3 U/mL to 312.6 U/mL (P < 0.01). The median antibody titer after the third vaccination of healthy individuals (n = 203) was 426.4 U/mL, which was comparable to that of patients (P = 0.2). The antibody titer after the third mRNA vaccination increased even in patients whose first two mRNA vaccinations failed. These findings suggest that allo-HSCT recipients should receive the mRNA vaccine regularly.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ministry of Health LaW: Visualizing the data: information on COVID-19 infections. https://covid19.mhlw.go.jp/en/ (2023). Accessed 12 May 2023
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–15. https://doi.org/10.1056/NEJMoa2034577.
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. https://doi.org/10.1056/NEJMoa2101765.
Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022;28(5):1063–71. https://doi.org/10.1038/s41591-022-01753-y.
Okamoto A, Fujigaki H, Iriyama C, Goto N, Yamamoto H, Mihara K, et al. CD19-positive lymphocyte count is critical for acquisition of anti-SARS-CoV-2 IgG after vaccination in B-cell lymphoma. Blood Adv. 2022;6(11):3230–3. https://doi.org/10.1182/bloodadvances.2021006302.
Terao T, Yamashita T, Fukumoto A, Kamura Y, Ikeda D, Kuzume A, et al. Low clinical protective response to SARS-CoV-2 mRNA COVID-19 vaccine in patients with multiple myeloma. Int J Hematol. 2022;115(5):737–47. https://doi.org/10.1007/s12185-022-03300-4.
Chang A, Akhtar A, Linderman SL, Lai L, Orellana-Noia VM, Valanparambil R, et al. Humoral responses against SARS-CoV-2 and variants of concern after mRNA vaccines in patients with non-Hodgkin lymphoma and chronic lymphocytic leukemia. J Clin Oncol. 2022;40(26):3020–31. https://doi.org/10.1200/jco.22.00088.
Lee A, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632. https://doi.org/10.1136/bmj-2021-068632.
Mittelman M, Magen O, Barda N, Dagan N, Oster HS, Leader A, et al. Effectiveness of the BNT162b2mRNA COVID-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood. 2022;139(10):1439–51. https://doi.org/10.1182/blood.2021013768.
Mori Y, Uchida N, Harada T, Katayama Y, Wake A, Iwasaki H, et al. Predictors of impaired antibody response after SARS-CoV-2 mRNA vaccination in hematopoietic cell transplant recipients: a Japanese multicenter observational study. Am J Hematol. 2023;98(1):102–11. https://doi.org/10.1002/ajh.26769.
Toya T, Atsuta Y, Sanada T, Honda T, Sadato D, Sekiya N, et al. Attenuated humoral response against SARS-CoV-2 mRNA vaccination in allogeneic stem cell transplantation recipients. Cancer Sci. 2023;114(2):586–95. https://doi.org/10.1111/cas.15603.
Maillard A, Redjoul R, Klemencie M, Labussière Wallet H, Le Bourgeois A, D‘Aveni M, et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood. 2022;139(1):134–7. https://doi.org/10.1182/blood.2021014232.
Khan QJ, Bivona CR, Martin GA, Zhang J, Liu B, He J, et al. Evaluation of the durability of the immune humoral response to COVID-19 vaccines in patients with cancer undergoing treatment or who received a stem cell transplant. JAMA Oncol. 2022;8(7):1053–8. https://doi.org/10.1001/jamaoncol.2022.0752.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. https://doi.org/10.1038/bmt.2012.244.
Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines -a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–79. https://doi.org/10.1038/nrd.2017.243.
Demaret J, Corroyer-Simovic B, Alidjinou EK, Goffard A, Trauet J, Miczek S, et al. Impaired functional T-cell response to SARS-CoV-2 after two doses of BNT162b2 mRNA vaccine in older people. Front Immunol. 2021;12:778679. https://doi.org/10.3389/fimmu.2021.778679.
Wingert A, Pillay J, Gates M, Guitard S, Rahman S, Beck A, et al. Risk factors for severity of COVID-19: a rapid review to inform vaccine prioritisation in Canada. BMJ Open. 2021;11(5):e044684. https://doi.org/10.1136/bmjopen-2020-044684.
Cook LB, O’Dell G, Vourvou E, Palanicawandar R, Marks S, Milojkovic D, et al. Third primary SARS-CoV-2 mRNA vaccines enhance antibody responses in most patients with haematological malignancies. Nat Commun. 2022;13(1):6922. https://doi.org/10.1038/s41467-022-34657-z.
Suzuki T, Kusumoto S, Kamezaki Y, Hashimoto H, Nishitarumizu N, Nakanishi Y, et al. A comprehensive evaluation of humoral immune response to second and third SARS-CoV-2 mRNA vaccination in patients with malignant lymphoma. Int J Hematol. 2023. https://doi.org/10.1007/s12185-023-03550-w.
Kuse N, Zhang Y, Chikata T, Nguyen HT, Oka S, Gatanaga H, et al. Long-term memory CD8(+) T cells specific for SARS-CoV-2 in individuals who received the BNT162b2 mRNA vaccine. Nat Commun. 2022;13(1):5251. https://doi.org/10.1038/s41467-022-32989-4.
Li C, Lee A, Grigoryan L, Arunachalam PS, Scott MKD, Trisal M, et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat Immunol. 2022;23(4):543–55. https://doi.org/10.1038/s41590-022-01163-9.
Acknowledgements
The authors thank Sayoko Ogata and Saori Takagi for their laboratory work.
Funding
This work was supported by grants from the Japan Society for the Promotion of Science (JSPS) KAKENHI (22k08501 to S.T.).
Author information
Authors and Affiliations
Contributions
ET, ST, AO, MM, and AT designed the study; ST, AO, KM, MS, TM, TG, YO, TN, NF, KO, RH, MM, and AT collected patient blood samples and clinical data; HF and KS measured anti-SARS-CoV-2 antibody titers; ET and ST performed statistical analysis; ET and ST generated figures and tables; ET, ST, AO, MM, AT, and HK wrote the paper. All the authors participated in discussions and interpretation of the data and results.
Corresponding author
Ethics declarations
Conflict of interest
H.F. and K.S. belong to an endowed department sponsored by FUJIFILIM Wako Pure Chemical Corporation. H.F. received a lecture fee from FUJIFILM Wako Pure Chemical Corporation. K.S. received research funding from FUJIFILM Wako Chemical Corporation. A.T. received research funding from Chugai Pharmaceutical, Astellas Pharma, Eisai, Otsuka Pharmaceutical, Ono Pharmaceutical, Kyowa Kirin, Shionogi, Sumitomo Dainippon Pharma, Taiho Pharmaceutical, Takeda Pharmaceutical, Teijin, Nippon Shinyaku, Nihon Pharmaceutical, Pfizer Japan, Mochida Pharmaceutical, Yakult Honsha, and Perseus Proteomics; and lecture fees from Chugai Pharmaceutical, Kyowa Kirin, Eisai, Takeda Pharmaceutical, Astellas Pharma, Nippon Shinyaku, Janssen Pharmaceutical, Zenyaku Kogyo, Abbvie GK, Bristol Myers Squibb, and SymBio Pharmaceutical. H. Kiyoi received research funding from FUJIFILM, Kyowa Kirin, Bristol Myers Squibb, Otsuka, Perseus Proteomics, Daiichi Sankyo, Abbvie, CURED, Astellas Pharma, Chugai, Zenyaku Kogyo, Nippon Shinyaku, Eisai, Takeda, Sumitomo Pharma, and Sanofi, and honoraria from Abbvie, Chugai, Astellas Pharma, and Novartis. The other authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Takagi, E., Terakura, S., Fujigaki, H. et al. Antibody response after third dose of COVID-19 mRNA vaccination in allogeneic hematopoietic stem cell transplant recipients is comparable to that in healthy counterparts. Int J Hematol 118, 462–471 (2023). https://doi.org/10.1007/s12185-023-03648-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-023-03648-1