Abstract
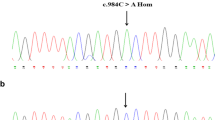
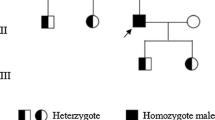
Inherited factor XIII (FXIII) deficiency is an extremely rare and under-diagnosed autosomal recessive inherited coagulopathy, which is caused by genetic defects in the F13A1 or F13B gene. More than 200 genetic mutations have been identified since the first case of inherited FXIII deficiency was reported. This study aimed to identify underlying gene mutations in a patient with inherited FXIII deficiency who presented with recurrent intracerebral hemorrhage. Levels of plasma FXIII-A antigen were measured, F13A1 and F13B genes were sequenced, mutation information was analyzed, and the mutated protein structure was predicted using bioinformatics methods. Molecular genetic analysis identified four mutations of FXIII-related genes in the proband, including three previously reported mutations inherited from his parents (c.631G>A, p.Gly210Arg and c.1687G>A, p.Gly562Arg of F13A1 gene and c.344G>A, p.Arg115His of F13B gene) and a novel spontaneous mutation of F13A1 gene (c.2063C>G, p.Ser687Cys). Molecular structural modeling demonstrated that the novel Ser687Cys mutation may cause changes in the spatial structure of FXIII-A and increase its instability. In conclusion, we identified a novel and likely pathogenic mutation of the F13A1 gene, which enriched the gene mutation spectrum of inherited FXIII deficiency. The findings may provide promising targets for diagnosis and treatment of inherited FXIII deficiency.





Similar content being viewed by others
Data availability
Research data of the study are available from the corresponding author on reasonable request.
References
Yee VC, Pedersen LC, Le Trong I, Bishop PD, Stenkamp RE, Teller DC. Three-dimensional structure of a transglutaminase: human blood coagulation factor XIII. Proc Natl Acad Sci USA. 1994;91(15):7296–300.
Schroeder V, Kohler HP. Factor XIII: structure and function. Semin Thromb Hemost. 2016;42(4):422–8.
Ichinose A, McMullen BA, Fujikawa K, Davie EW. Amino acid sequence of the b subunit of human factor XIII, a protein composed of ten repetitive segments. Biochemistry. 1986;25(16):4633–8.
Durda MA, Wolberg AS, Kerlin BA. State of the art in factor XIII laboratory assessment. Transfus Apher Sci. 2018;57(6):700–4.
Inbal A, Lubetsky A, Krapp T, Castel D, Shaish A, Dickneitte G, et al. Impaired wound healing in factor XIII deficient mice. Thromb Haemost. 2005;94(2):432–7.
Dardik R, Loscalzo J, Inbal A. Factor XIII (FXIII) and angiogenesis. J Thromb Haemost. 2006;4(1):19–25.
Inbal A, Muszbek L. Coagulation factor deficiencies and pregnancy loss. Semin Thromb Hemost. 2003;29(2):171–4.
Soendergaard C, Kvist PH, Seidelin JB, Nielsen OH. Tissue-regenerating functions of coagulation factor XIII. J Thromb Haemost. 2013;11(5):806–16.
Muszbek L, Katona É. Diagnosis and management of congenital and acquired FXIII deficiencies. Semin Thromb Hemost. 2016;42(4):429–39.
Yan MTS, Rydz N, Goodyear D, Sholzberg M. Acquired factor XIII deficiency: a review. Transfus Apher Sci. 2018;57(6):724–30.
Dorgalaleh A, Rashidpanah J. Blood coagulation factor XIII and factor XIII deficiency. Blood Rev. 2016;30(6):461–75.
Dorgalaleh A, Naderi M, Shamsizadeh M. Morbidity and mortality in a large number of Iranian patients with severe congenital factor XIII deficiency. Ann Hematol. 2016;95(3):451–5.
Naderi M, Dorgalaleh A, Alizadeh S, Tabibian S, Hosseini S, Shamsizadeh M, et al. Clinical manifestations and management of life-threatening bleeding in the largest group of patients with severe factor XIII deficiency. Int J Hematol. 2014;100(5):443–9.
Karimi M, Bereczky Z, Cohan N, Muszbek L. Factor XIII deficiency. Semin Thromb Hemost. 2009;35(4):426–38.
Lak M, Peyvandi F, Ali Sharifian A, Karimi K, Mannucci PM. Pattern of symptoms in 93 Iranian patients with severe factor XIII deficiency. J Thromb Haemost. 2003;1(8):1852–3.
Peyvandi F, Palla R, Menegatti M, Siboni SM, Halimeh S, Faeser B, et al. Coagulation factor activity and clinical bleeding severity in rare bleeding disorders: results from the European Network of Rare Bleeding Disorders. J Thromb Haemost. 2012;10(4):615–21.
Alavi SER, Jalalvand M, Assadollahi V, Tabibian S, Dorgalaleh A. Intracranial hemorrhage: a devastating outcome of congenital bleeding disorders-prevalence, diagnosis, and management, with a special focus on congenital factor XIII deficiency. Semin Thromb Hemost. 2018;44(3):267–75.
Kohler HP, Ichinose A, Seitz R, Ariens RA, Muszbek L. Diagnosis and classification of factor XIII deficiencies. J Thromb Haemost. 2011;9(7):1404–6.
Karimi M, Peyvandi F, Naderi M, Shapiro A. Factor XIII deficiency diagnosis: challenges and tools. Int J Lab Hematol. 2018;40(1):3–11.
Nugent D. Corifact™/Fibrogammin® P in the prophylactic treatment of hereditary factor XIII deficiency: results of a prospective, multicenter, open-label study. Thromb Res. 2012;130(Suppl 2):S12–4.
Ashley C, Chang E, Davis J, Mangione A, Frame V, Nugent DJ. Efficacy and safety of prophylactic treatment with plasma-derived factor XIII concentrate (human) in patients with congenital factor XIII deficiency. Haemophilia. 2015;21(1):102–8.
Carcao M, Altisent C, Castaman G, Fukutake K, Kerlin BA, Kessler C, et al. Recombinant FXIII (rFXIII-A2) prophylaxis prevents bleeding and allows for surgery in patients with congenital FXIII A-subunit deficiency. Thromb Haemost. 2018;118(3):451–60.
Jain S, Acharya SS. Management of rare coagulation disorders in 2018. Transfus Apher Sci. 2018;57(6):705–12.
Franchini M, Marano G, Mengoli C, Piccinini V, Pupella S, Vaglio S, et al. Inhibitors in patients with congenital bleeding disorders other than hemophilia. Semin Thromb Hemost. 2018;44(6):595–603.
Duckert F, Jung E, Shmerling DH. A hitherto undescribed congenital haemorrhagic diathesis probably due to fibrin stabilizing factor deficiency. Thromb Diath Haemorrh. 1960;5:179–86.
Carcao M, Fukutake K, Inbal A, Kerlin B, Lassila R, Oldenburg J, et al. Developing the first recombinant factor XIII for congenital factor XIII deficiency: clinical challenges and successes. Semin Thromb Hemost. 2017;43(1):59–68.
Thomas A, Biswas A, Dodt J, Philippou H, Hethershaw E, Ensikat HJ, et al. Coagulation factor XIIIA subunit missense mutations affect structure and function at the various steps of factor XIII action. Hum Mutat. 2016;37(10):1030–41.
Vysokovsky A, Rosenberg N, Dardik R, Seligsohn U, Inbal A. Effect of four missense mutations in the factor XIII A-subunit gene on protein stability: studies with recombinant proteins. Blood Coagul Fibrinolysis. 2006;17(2):125–30.
Vysokovsky A, Saxena R, Landau M, Zivelin A, Eskaraev R, Rosenberg N, et al. Seven novel mutations in the factor XIII A-subunit gene causing hereditary factor XIII deficiency in 10 unrelated families. J Thromb Haemost. 2004;2(10):1790–7.
Takahashi N, Tsukamoto H, Umeyama H, Castaman G, Rodeghiero F, Ichinose A. Molecular mechanisms of type II factor XIII deficiency: novel Gly562-Arg mutation and C-terminal truncation of the A subunit cause factor XIII deficiency as characterized in a mammalian expression system. Blood. 1998;91(8):2830–8.
Komanasin N, Catto AJ, Futers TS, van Hylckama VA, Rosendaal FR, Ariëns RA. A novel polymorphism in the factor XIII B-subunit (His95Arg): relationship to subunit dissociation and venous thrombosis. J Thromb Haemost. 2005;3(11):2487–96.
Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011;91(3):931–72.
Anwar R, Miloszewski KJ, Markham AF. Identification of a large deletion, spanning exons 4 to 11 of the human factor XIIIA gene, in a factor XIII-deficient family. Blood. 1998;91(1):149–53.
Jiao WY, Wu JS, Ding QL, Wang XF, Xu XC, Ding KY, et al. Identification of a novel mutation of F (13) A gene in a pedigree with factor XIII deficiency. Zhonghua Xue Ye Xue Za Zhi. 2007;28(9):598–601.
Ivaskevicius V, Windyga J, Baran B, Schroeder V, Junen J, Bykowska K, et al. Phenotype-genotype correlation in eight Polish patients with inherited Factor XIII deficiency: identification of three novel mutations. Haemophilia. 2007;13(5):649–57.
Otaki M, Inaba H, Shinozawa K, Fujita S, Amano K, Fukutake K. Characterization of a large deletion that leads to congenital factor XIII deficiency. Rinsho Byori. 2008;56(3):187–94.
Ma QL, Zhou KY, Zhou P, Cai WW. Identification of a novel large deletion of factor subunit A mRNA associated with hereditary factor deficiency. Zhonghua Xue Ye Xue Za Zhi. 2012;33(4):299–302.
Thomas A, Ivaškevičius V, Zawadzki C, Goudemand J, Biswas A, Oldenburg J. Characterization of a novel large deletion caused by double-stranded breaks in 6-bp microhomologous sequences of intron 11 and 12 of the F13A1 gene. Hum Genome Var. 2016;3:15059.
Ma S, Chen C, Liang Q, Wu X, Wang X, Wu W, et al. Phenotype and genotype of FXIII deficiency in two unrelated probands: identification of a novel F13A1 large deletion mediated by complex rearrangement. Orphanet J Rare Dis. 2019;14(1):182.
Shanbhag S, Ghosh K, Shetty S. Genetic basis of severe factor XIII deficiency in a large cohort of Indian patients: identification of fourteen novel mutations. Blood Cells Mol Dis. 2016;57:81–4.
Kershaw G. Detection and measurement of factor inhibitors. In: Favaloro EJ, Lippi G, editors. Hemostasis and thrombosis: methods and protocols. New York: Springer; 2017. pp. 295–304.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24.
Acknowledgements
Zi Sheng and Yunhai Fang designed the research; Jun Peng, Jihua Qiu, and Xinsheng Zhang conducted the research; Lijie Yan collected the clinical data; Lijie Yan and Tiantian Wang analyzed the data and drafted the paper. All authors revised and approved the final version of the manuscript. This study was funded by the National Natural Science Foundation of China (82000126, 91942306, and 81770133), Shandong Medical and Health Science and Technology Development Program (202103040601), and China Postdoctoral Science Foundation (2020M672075). The authors have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors state that they have no interests which might be perceived as posing a conflict or bias.
Ethics approval statement
Ethics approval (No. KYLL-202205-023-1) was obtained from The Medical Ethics Committee of Qilu Hospital, Shandong University.
Patient consent statement
All participants gave informed consent to our research in accordance with the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The work was done in Qilu Hospital of Shandong University and Shandong Hemophilia Treatment Center.
Supplementary Information
Below is the link to the electronic supplementary material.
12185_2023_3594_MOESM1_ESM.tiff
Supplementary file1 (TIFF 26384 kb) Supplementary Fig. 1 Three-dimensional structure of mutant protein predicted by AlphaFold. A Mutant protein structure when Ser687Cys exists together with Gly210Arg in one allele. B Mutant protein structure when Ser687Cys exists together with Gly562Arg in one allele. C Local protein structure caused by Ser687Cys mutation. Yellow dashed lines represent hydrogen bonds. Hydrogen bond lengths are marked with black numbers. Residues and corresponding colors are as follows: Arg210-blue, Arg562-magentas, Cys687-orange
12185_2023_3594_MOESM2_ESM.tiff
Supplementary file2 (TIFF 26384 kb) Supplementary Fig. 1 Three-dimensional structure of mutant protein predicted by AlphaFold. A Mutant protein structure when Ser687Cys exists together with Gly210Arg in one allele. B Mutant protein structure when Ser687Cys exists together with Gly562Arg in one allele. C Local protein structure caused by Ser687Cys mutation. Yellow dashed lines represent hydrogen bonds. Hydrogen bond lengths are marked with black numbers. Residues and corresponding colors are as follows: Arg210-blue, Arg562-magentas, Cys687-orange
12185_2023_3594_MOESM3_ESM.docx
Supplementary file3 (TIFF 26384 kb) Supplementary Fig. 1 Three-dimensional structure of mutant protein predicted by AlphaFold. A Mutant protein structure when Ser687Cys exists together with Gly210Arg in one allele. B Mutant protein structure when Ser687Cys exists together with Gly562Arg in one allele. C Local protein structure caused by Ser687Cys mutation. Yellow dashed lines represent hydrogen bonds. Hydrogen bond lengths are marked with black numbers. Residues and corresponding colors are as follows: Arg210-blue, Arg562-magentas, Cys687-orange
12185_2023_3594_MOESM4_ESM.tiff
Supplementary file4 (DOCX 15 kb) Supplementary Table 1 Sequencing results of F13A1 and F13B genes in the patient'sparents
About this article
Cite this article
Yan, L., Wang, T., Qiu, J. et al. Identification of a novel mutation in the factor XIII A subunit in a patient with inherited factor XIII deficiency. Int J Hematol 118, 26–35 (2023). https://doi.org/10.1007/s12185-023-03594-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-023-03594-y