Abstract
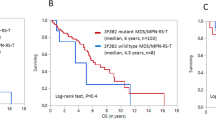
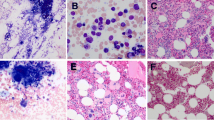
Myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T) is a rare disease, which presents with features of myelodysplastic syndromes with ring sideroblasts and essential thrombocythemia, as well as anemia and marked thrombocytosis. SF3B1 and JAK2 mutations are often found in patients, and are associated with their specific clinical features. This study was a retrospective analysis of 34 Japanese patients with MDS/MPN-RS-T. Median age at diagnosis was 77 (range, 51–88) years, and patients had anemia (median hemoglobin: 9.0 g/dL) and thrombocytosis (median platelet count: 642 × 109/L). Median overall survival was 70 (95% confidence interval: 68-not applicable) months during the median follow-up period of 26 (range: 0–91) months. A JAK2V617F mutation was detected in 46.2% (n = 12) of analyzed patients (n = 26), while an SF3B1 mutation was detected in 87.5% (n = 7) of analyzed patients (n = 8). Like those with myelodysplastic syndromes or myeloproliferative neoplasms, patients often received erythropoiesis-stimulating agents and aspirin to improve anemia and prevent thrombosis. This study, which was the largest to describe the real-world characteristics of Japanese patients with MDS/MPN-RS-T, showed that the patients had similar characteristics to those in western countries.


Similar content being viewed by others
References
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues revised. 4th ed. Lyon, France: IARC; 2017.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
Remacha AF, Nomdedéu JF, Puget G, Estivill C, Sarda MP, Canals C, et al. Occurrence of the JAK2 V617F mutation in the WHO provisional entity: myelodysplastic/myeloproliferative disease, unclassifiable-refractory anemia with ringed sideroblasts associated with marked thrombocytosis. Haematologica. 2006;91(5):719–20.
Gattermann N, Billiet J, Kronenwett R, Zipperer E, Germing U, Nollet F, et al. High frequency of the JAK2 V617F mutation in patients with thrombocytosis (platelet count>600x109/L) and ringed sideroblasts more than 15% considered as MDS/MPD, unclassifiable. Blood. 2007;109(3):1334–5.
Patnaik MM, Lasho TL, Hodnefield JM, Knudson RA, Ketterling RP, Garcia-Manero G, et al. SF3B1 mutations are prevalent in myelodysplastic syndromes with ring sideroblasts but do not hold independent prognostic value. Blood. 2012;119(2):569–72.
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the world health organization classification of haematolymphoid tumours myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19.
Broséus J, Alpermann T, Wulfert M, Florensa Brichs L, Jeromin S, Lippert E, et al. Age, JAK2(V617F) and SF3B1 mutations are the main predicting factors for survival in refractory anaemia with ring sideroblasts and marked thrombocytosis. Leukemia. 2013;27(9):1826–31.
Broseus J, Florensa L, Zipperer E, Schnittger S, Malcovati L, Richebourg S, et al. Clinical features and course of refractory anemia with ring sideroblasts associated with marked thrombocytosis. Haematologica. 2012;97(7):1036–41.
Mangaonkar AA, Lasho TL, Ketterling RP, Reichard KK, Gangat N, Al-Kali A, et al. Myelodysplastic/myeloproliferative neoplasms with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T): Mayo-Moffitt collaborative study of 158 patients. Blood Cancer J. 2022;12(2):26.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Inano T, Araki M, Morishita S, Imai M, Yasuda H, Nitta H, et al. JAK2 exon 12 mutation in myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis: not an exclusive mutation to polycythaemia vera. Br J Haematol. 2019;187(1):e27–31.
Hashimoto Y, Ito T, Gotoh A, Nakamae M, Kimura F, Koike M, et al. Clinical characteristics, prognostic factors, and outcomes of patients with essential thrombocythemia in Japan: the JSH-MPN-R18 study. Int J Hematol. 2022;115(2):208–21.
Tasaka T, Tohyama K, Kishimoto M, Ohyashiki K, Mitani K, Hotta T, et al. Myelodysplastic syndrome with chromosome 5 abnormalities: a nationwide survey in Japan. Leukemia. 2008;22(10):1874–81.
Kobayashi T, Nannya Y, Ichikawa M, Oritani K, Kanakura Y, Tomita A, et al. A nationwide survey of hypoplastic myelodysplastic syndrome (a multicenter retrospective study). Am J Hematol. 2017;92(12):1324–32.
Patnaik MM, Lasho TL, Finke CM, Hanson CA, King RL, Ketterling RP, et al. Predictors of survival in refractory anemia with ring sideroblasts and thrombocytosis (RARS-T) and the role of next-generation sequencing. Am J Hematol. 2016;91(5):492–8.
Jeromin S, Haferlach T, Weissmann S, Meggendorfer M, Eder C, Nadarajah N, et al. Refractory anemia with ring sideroblasts and marked thrombocytosis cases harbor mutations in SF3B1 or other spliceosome genes accompanied by JAK2V617F and ASXL1 mutations. Haematologica. 2015;100(4):e125–7.
Malcovati L, Cazzola M. Refractory anemia with ring sideroblasts. Best Pract Res Clin Haematol. 2013;26(4):377–85.
Montalban-Bravo G, Garcia-Manero G. MDS/MPN-RS-T justified inclusion as a unique disease entity? Best Pract Res Clin Haematol. 2020;33(2): 101147.
Schnittger S, Bacher U, Haferlach C, Dengler R, Kröber A, Kern W, et al. Detection of an MPLW515 mutation in a case with features of both essential thrombocythemia and refractory anemia with ringed sideroblasts and thrombocytosis. Leukemia. 2008;22(2):453–5.
Antelo G, Mangaonkar AA, Coltro G, Buradkar A, Lasho TL, Finke C, et al. Response to erythropoiesis-stimulating agents in patients with WHO-defined myelodysplastic syndrome/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T). Br J Haematol. 2020;189(3):e104–8.
Fenaux P, Platzbecker U, Mufti GJ, Garcia-Manero G, Buckstein R, Santini V, et al. Luspatercept in patients with lower-risk myelodysplastic syndromes. N Engl J Med. 2020;382(2):140–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
YE reports grants or contracts from PharmaEssentia Japan K.K., Meiji Seika Pharma and AbbVie G.K.; honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from PharmaEssentia Japan K.K., Takeda Pharmaceutical Co., Ltd. and Novartis Pharma K.K.; and participated on a data safety monitoring board or advisory board of Novartis Pharma K.K. and PharmaEssentia Japan K.K. TO reports grants or contracts from PharmaEssentia Japan K.K. and Meiji Seika Pharma. YH reports honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Takeda Pharmaceutical Co., Ltd. and Novartis Pharma K.K. SJ reports supports for the present manuscript from Otsuka Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Takeda Pharmaceutical Co., Ltd., Novartis Pharma K.K., Sumitomo Pharma Co., Ltd., and Bristol-Myers Squibb K.K.; grants or contracts from FUJIFILM Wako Pure Chemical Corporation, Fuso Pharmaceutical, Pfizer Japan Inc., PharmaEssentia Japan K.K, Shire International GmbH, Perseus Proteomics Inc., Meiji Seika Pharma. SS reports grants or contracts from PharmaEssentia Japan K.K. and Meiji Seika Pharma. KU reports grants or contracts from Amgen-Astellas Biopharma K.K., Otsuka Pharmaceutical Co., LTD., Apellis Pharmaceuticals, Inc., SynBio Pharmaceuticals Ltd., Takeda Pharmaceutical Co., Ltd., Nippon-Shinyaku Co. Ltd., Novartis Pharma K.K., AbbVie G.K., Janssen Pharmaceutical K.K., Bristol-Myers Squibb K.K., Ono Pharmaceutical Co., LTD., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd, MSD K.K., Astellas Pharma Inc., Alexion Pharmaceuticals, Inc., Kyowa Kirin Co., Ltd., Gilead Sciences, Inc., Pfizer Japan Inc., Incyte Biosciences Japan G.K., SynBio Pharmaceuticals Ltd., Celgene K.K., Sumitomo Dainippon Pharma Co., Ltd., Mundipharma K.K., Yakult Honsha Co., and Eisai Co., Ltd.; consulting fees from Alexion Pharmaceuticals, Inc., SynBio Pharmaceuticals Ltd., Nippon-Shinyaku Co. Ltd., Otsuka Pharmaceutical Co., LTD., Chugai Pharmaceutical Co., Ltd., Sanofi K.K., Takeda Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Astellas Pharma Inc., SOBI, and Alnylam Japan; and honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis Pharma K.K., Astellas Pharma Inc., Alexion Pharmaceuticals, Inc., Eisai Co., Ltd., MSD, Otsuka Pharmaceutical Co., LTD., Ono Pharmaceutical Co., LTD., Kyowa Kirin Co., Ltd., Celgene K.K., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon-Shinyaku Co. Ltd., PharmaEssentia Japan K.K., Bristol-Myers Squibb K.K., Yakult Honsha Co., Sanofi K.K., Pfizer Japan Inc., AbbVie G.K., and Chugai Pharmaceutical Co., Ltd. MN reports honoraria for lectures from Takeda Pharmaceutical Co., Ltd., Nippon-Shinyaku Co. Ltd., Novartis Pharma K.K. and Bristol-Myers Squibb K.K. YU participated on a data safety monitoring board or advisory board of Sanofi K.K. and Otsuka Pharmaceutical Co., LTD. JY reports honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Takeda Pharmaceutical Co., Ltd. and Chugai Pharmaceutical Co., Ltd. TK reports consulting fees from Sanwa Kagaku Kenkyusyo Co., LTD.; and honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis Pharma K.K., Takeda Pharmaceutical CO., LTD., Ono Pharmaceutical Co., LTD., Otsuka Pharmaceutical C0., LTD., Nippon-Shinyaku Co., LTD. and Bristol-Myers Squibb K.K. MA reports grants or contracts from Century Therapeutics and Daiichi Sankyo Co., Ltd; and honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie G.K., Sumitomo Pharma Co., Ltd., Novartis Pharma K.K. and Sanofi K.K. NK reports grants or contracts from Otsuka Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Sumitomo Pharma Co., Ltd., PharmaEssentia Japan K.K., Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Perseus Proteomics Inc. and Meiji Seika Pharma Co., Ltd.; consulting fees from Japan Tobacco Inc. and PharmaEssentia Japan K.K.; honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis Pharma K.K. and Takeda Pharmaceutical Co., Ltd.; and is a board member of PharmaEssentia Japan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Edahiro, Y., Ochiai, T., Hashimoto, Y. et al. Clinical characteristics of Japanese patients with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis. Int J Hematol 118, 47–53 (2023). https://doi.org/10.1007/s12185-023-03592-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-023-03592-0