Abstract
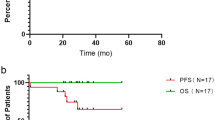
Chemotherapy with cytarabine, vincristine (VCR), and prednisolone has achieved low mortality rates in pediatric patients with Langerhans cell histiocytosis (LCH). However, relapse rates remain high, making event-free survival (EFS) rates unsatisfactory. A nationwide clinical trial, LCH-12, tested a modified protocol in which the early maintenance phase was intensified with increasing dosages of VCR. Patients newly diagnosed with multifocal bone (MFB) or multisystem (MS) LCH and aged < 20 years at diagnosis were enrolled between June 2012 and November 2017. Of the 150 eligible patients, 43 with MFB were treated for 30 weeks and 107 with MS LCH were treated for 54 weeks. One patient with MS LCH died of sepsis during the induction phase. The 3-year EFS rates among patients with MFB LCH, risk organ (RO)-negative MS LCH, and RO-positive MS LCH were 66.7% (95% confidential interval [CI], 56.5–77.0%), 66.1% (95% CI 52.9–76.4%), and 51.1% (95% CI 35.8–64.5%), respectively, similar to previously observed rates. EFS rates were significantly lower in patients with disease activity scores > 6 than in those with scores ≤ 6. The strategy that included more intense treatment with VCR was not effective. Other strategies are required to improve outcomes in patients with pediatric LCH.




Similar content being viewed by others
Data Availability
Due to ethical considerations, individual participant data will not be available.
References
Rodriguez-Galindo C, Allen CE. Langerhans cell histiocytosis. Blood. 2020;135:1319–31.
Morimoto A, Oh Y, Shioda Y, Kudo K, Imamura T. Recent advances in Langerhans cell histiocytosis. Pediatr Int. 2014;56:451–61.
Minkov M, Grois N, Heitger A, Pötschger U, Westermeier T, Gadner H. Treatment of multisystem Langerhans cell histiocytosis. Results of the DAL-HX 83 and DAL-HX 90 studies. DAL-HX Study Group. Klin Padiatr. 2000;212:139–44.
Titgemeyer C, Grois N, Minkov M, Flucher-Wolfram B, Gatterer-Menz I, Gadner H. Pattern and course of single-system disease in Langerhans cell histiocytosis data from the DAL-HX 83- and 90-study. Med Pediatr Oncol. 2001;37:108–14.
Gadner H, Grois N, Arico M, Broadbent V, Ceci A, Jakobson A, Histiocyte Society, et al. A randomized trial of treatment for multisystem Langerhans’ cell histiocytosis. J Pediatr. 2001;138:728–34.
Gadner H, Grois N, Pötschger U, Minkov M, Aricò M, Braier J, Histiocyte Society, et al. Improved outcome in multisystem Langerhans cell histiocytosis is associated with therapy intensification. Blood. 2008;111:2556–62.
Gadner H, Minkov M, Grois N, Pötschger U, Thiem E, Aricò M, Histiocyte Society, et al. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood. 2013;121:5006–14.
Morimoto A, Ikushima S, Kinugawa N, Ishii E, Kohdera U, Sako M, Japan Langerhans Cell Histiocytosis Study Group, et al. Improved outcome in the treatment of pediatric multifocal Langerhans cell histiocytosis: Results from the Japan Langerhans Cell Histiocytosis Study Group-96 protocol study. Cancer. 2006;107:613–9.
Morimoto A, Shioda Y, Imamura T, Kudo K, Kawaguchi H, Sakashita K, et al. Intensified and prolonged therapy comprising cytarabine, vincristine and prednisolone improves outcome in patients with multisystem Langerhans cell histiocytosis: results of the Japan Langerhans Cell Histiocytosis Study Group-02 Protocol Study. Int J Hematol. 2016;104:99–109.
Morimoto A, Shioda Y, Imamura T, Kudo K, Kitoh T, Kawaguchi H, Japan LCH Study Group, et al. Intensification of induction therapy and prolongation of maintenance therapy did not improve the outcome of pediatric Langerhans cell histiocytosis with single-system multifocal bone lesions: results of the Japan Langerhans Cell Histiocytosis Study Group-02 Protocol Study. Int J Hematol. 2018;108:192–8.
Sakamoto K, Morimoto A, Shioda Y, Imamura T, Imashuku S, on behalf of the Japan LCH Study Group (JLSG). Relapses of multisystem/multifocal bone Langerhans cell histiocytosis in paediatric patients: data analysis from the JLSG-96/02 study. Br J Haematol. 2022. https://doi.org/10.1111/bjh.18583.
Sakamoto K, Morimoto A, Shioda Y, Imamura T, Imashuku S. Central diabetes insipidus in pediatric patients with Langerhans cell histiocytosis: Results from the JLSG-96/02 studies. Pediatr Blood Cancer. 2019;66: e27454.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. p. 358–60.
Minkov M, Grois N, McClain K, Nanduri V, Rodriguez-Galindo C, Simonitsch-Klupp I, et al. Langerhans cell histiocytosis: Histocyte Society evaluation and treatment guidelines. April 2009. http://www.hematologie-amc.nl/bestanden/hematologie/bijlagennietinDBS/SocietyLCHTreatmentGuidelines.PDF. Accessed 1 Apr 2022.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Donadieu J, Piguet C, Bernard F, Barkaoui M, Ouache M, Bertrand Y, et al. A new clinical score for disease activity in Langerhans cell histiocytosis. Pediatr Blood Cancer. 2004;43:770–6.
Egeler RM, Favara BE, van Meurs M, Laman JD, Claassen E. Differential in situ cytokine profiles of Langerhans-like cells and T cells in Langerhans cell histiocytosis: abundant expression of cytokines relevant to disease and treatment. Blood. 1999;94:4195–201.
Said R, Tsimberidou AM. Pharmacokinetic evaluation of vincristine for the treatment of lymphoid malignancies. Expert Opin Drug Metab Toxicol. 2014;10:483–94.
Murphy T, Yee KWL. Cytarabine and daunorubicin for the treatment of acute myeloid leukemia. Expert Opin Pharmacother. 2017;18:1765–80.
Tzortzatou-Stathopoulou F, Papadopoulou AL, Moschovi M, Botsonis A, Tsangaris GT. Low relapse rate in children with acute lymphoblastic leukemia after risk-directed therapy. J Pediatr Hematol Oncol. 2001;23:591–7.
Ronceray L, Pötschger U, Janka G, Gadner H, Minkov M, German Society for Pediatric Hematology and Oncology, Langerhans Cell Histiocytosis Study Group. Pulmonary involvement in pediatric-onset multisystem Langerhans cell histiocytosis: effect on course and outcome. J Pediatr. 2012;161:129–33.
Rigaud C, Barkaoui MA, Thomas C, Bertrand Y, Lambilliotte A, Miron J, et al. Langerhans cell histiocytosis: therapeutic strategy and outcome in a 30-year nationwide cohort of 1478 patients under 18 years of age. Br J Haematol. 2016;174:887–98.
Donadieu J, Larabi IA, Tardieu M, Visser J, Hutter C, Sieni E, et al. Vemurafenib for refractory multisystem Langerhans cell histiocytosis in children: an international observational study. J Clin Oncol. 2019;37:2857–65.
Sakamoto K, Morimoto A, Shioda Y, Imamura T, Imashuku S, Japan LCH Study Group (JLSG). Long-term complications in uniformly treated paediatric Langerhans histiocytosis patients disclosed by 12 years of follow-up of the JLSG-96/02 studies. Br J Haematol. 2021;192:615–20.
Acknowledgements
We thank all of the patients, their families, physicians and staff in the Japanese Paediatric Leukaemia/Lymphoma Study Group and Japan Children’s Cancer Group who contributed to this study.
Funding
This work was supported by the Ministry of Health, Labor, and Welfare, Japan (Grant number: Research on Measures for Intractable Disease H22-General-072, H24-General-076 and H26-General-068 to A. Morimoto) and the Japan Agency for Medical Research and Development, Japan (Grant number: 15ek0109055h0202 and 16ek0109055h0203 to A. Morimoto, and Grant 193 number: 20ck0106605h0001 to Y. Shioda).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12185_2023_3568_MOESM1_ESM.tif
Outcomes of patients in the trial treatment group. MFB, multifocal bone; MS, multisystem; RO, risk organ involvement; GR, good response; PR, partial response; NR, no response; PD, progressive disease; AD-p, active disease progression (TIF 322 KB)
About this article
Cite this article
Morimoto, A., Shioda, Y., Kudo, K. et al. Intensification of treatment with vinca alkaloid does not improve outcomes in pediatric patients with Langerhans cell histiocytosis: results from the JPLSG LCH-12 study. Int J Hematol 118, 107–118 (2023). https://doi.org/10.1007/s12185-023-03568-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-023-03568-0