Abstract
Background
Recombinant factor VIII-Fc (rFVIIIFc) became available in Taiwan in 2018. Before this date, no people with hemophilia A (PwHA) were enrolled in a clinical trial of rFVIIIFc. We investigated changes in bleeding outcomes and product utilization in PwHA switching from rFVIII to rFVIIIFc.
Methods
Data were collected for Taiwanese PwHA (severe-type) who switched from rFVIII to rFVIIIFc, including annualized bleeding rate (ABR) and weekly dose consumption 12 months pre-switch and > 6 months post-switch.
Results
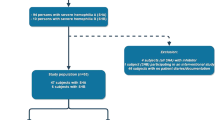
The 51 patients were divided into 3 groups according to their pre-switch treatment: on-demand treatment, intermittent periodic prophylaxis, and regular prophylaxis. In every group, the post-switch median ABR was significantly reduced, with no significant differences between groups. Meanwhile, the post-switch median weekly dose of each group was significantly increased. In 32 patients on pre-switch prophylaxis, switching brought a further reduction in median ABR, associated with a significant increase in median weekly dose. No adverse effects or novel inhibitor development were seen.
Conclusion
This is the first report from Asia on real-world experience of rFVIIIFc, showing that switching to rFVIIIFc prophylaxis led to further reduction in ABR and increase in weekly dose for all patient groups, even those on pre-switch rFVIII prophylaxis.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
References
Fischer K, van der Bom JG, Molho P, Negrier C, Mauser-Bunschoten EP, Roosendaal G, et al. Prophylactic vs. on-demand treatment strategies for severe haemophilia: a comparison of costs and long-term outcome. Haemophilia. 2002;8:745–52.
Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus Episodic Treatment to Prevent Joint Disease in Boys with Severe Hemophilia. N Engl J Med. 2007;357:535–44.
Hay CR. Prophylaxis in adults with haemophilia. Haemophilia. 2007;13(Suppl 2):10–5.
Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, et al. WFH guidelines for the management of hemophilia panelists and co-authors. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1–158.
Hermans C, Mancuso ME, Nolan B, Pasi KJ. Recombinant factor VIII Fc for the treatment of haemophilia A. Eur J Haematol. 2021;106(6):745–61.
Taki M, Fukutake K, Kobayashi M, Nagao A, Makioka H, Shiraishi M. Initial safety results from a prospective post-marketing surveillance study using rFVIIIFc in the real world setting in Japanese hemophilia A patients. Res Practice Thrombosis Haemostasis. 2017;1(Suppl1):738.
Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A. Subcommittee on Factor VIII, Factor IX and rare coagulation disorders of the scientific and standardization committee of the international society on thrombosis and hemostasis. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–9.
Chang CY, Li TY, Cheng SN, Pan RY, Wang HJ, Lin SY, et al. Prevalence and severity by age and other clinical correlates of haemophilic arthropathy of the elbow, knee and ankle among Taiwanese patients with haemophilia. Haemophilia. 2017;23(2):284–91.
Chen YC, Chang CY, Cheng SN, Pan RY, Shih YL, Li TY, et al. Evolution of congenital haemophilia care in Taiwan. J Formos Med Assoc. 2021. https://doi.org/10.1016/j.jfma.2021.07.017.
Thornburg CD, Duncan NA. Treatment adherence in hemophilia. Patient Prefer Adherence. 2017;11:1677–86.
Mahlangu J, Powell JS, Ragni MV, Chowdary P, Josephson NC, Pabinger I, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317–25.
Nolan B, Mahlangu J, Perry D, Young G, Liesner R, Konkle B, et al. Long-term safety and efficacy of recombinant factor VIII Fc fusion protein (rFVIIIFc) in subjects with haemophilia A. Haemophilia. 2016;22(1):72–80.
Young G, Mahlangu J, Kulkarni R, Nolan B, Liesner R, Pasi J, et al. Recombinant factor VIII Fc fusion protein for the prevention and treatment of bleeding in children with severe hemophilia A. J Thromb Haemost. 2015;13(6):967–77.
Wang C, Young G. Clinical use of recombinant factor VIII Fc and recombinant factor IX Fc in patients with haemophilia A and B. Haemophilia. 2018;24(3):414–9.
Giraud R, Delmotte N, Gensollen S, Roche M, Falaise C, Chambost H, et al. Recombinant factor VIII Fc fusion protein (rFVIIIFc) in real life: one-year clinical and economic outcomes. Drugs Real World Outcomes. 2021;8(4):527–35.
Brennan Y, Parikh S, McRae S, Tran H. The Australian experience with switching to extended half-life factor VIII and IX concentrates: on behalf of the Australian Haemophilia Centre Director’s Organisation. Haemophilia. 2020;26(3):529–35.
Wall C, Scott M, Xiang H, Palmer B, Collins P, Chowdary P, et al. Longitudinal Analysis of rFVIIIFc Use and Efficacy in the UK: A Report from the National Haemophilia Database [abstract]. Res Pract Thromb Haemost 2020;4 (Suppl 1). https://abstracts.isth.org/abstract/longitudinal-analysis-of-rfviiifc-use-and-efficacy-in-the-uk-a-report-from-the-national-haemophilia-database/. Accessed August 18, 2021
Holmström M, Olsson E, Astermark J, Axelsson M, Olsson A, Myrin Westesson L, et al. Real-world prophylactic usage of recombinant factor VIII Fc in Sweden: a report from the Swedish national registry for bleeding disorders. Haemophilia. 2021;27(4):e554–8.
Sun HL, Yang M, Poon MC, Lee A, Robinson KS, Sholzberg M, et al. Factor product utilization and health outcomes in patients with haemophilia A and B on extended half-life concentrates: a Canadian observational study of real-world outcomes. Haemophilia. 2021;27(5):751–9.
Tagliaferri A, Matichecchia A, Rivolta GF, Riccardi F, Quintavalle G, Benegiamo A, et al. Optimising prophylaxis outcomes and costs in haemophilia patients switching to recombinant FVIII-Fc: a single-centre real-world experience. Blood Transfus. 2020;18(5):374–85.
Ay C, Feistritzer C, Rettl J, Schuster G, Vavrovsky A, Perschy L, et al. Bleeding outcomes and factor utilization after switching to an extended half-life product for prophylaxis in haemophilia A in Austria. Sci Rep. 2021;11:12967.
Gouw SC, van der Bom JG, Marijke van den Berg H. Treatment-related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood. 2007;109(11):4648–54.
Peyvandi F, Mannucci PM, Garagiola I, El-Beshlawy A, Elalfy M, Ramanan V, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med. 2016;374(21):2054–64.
Acknowledgements
The authors would like to thank Dr. Vivek R. Sharma, Research Scientist and Medical Director at the Adult Hemophilia Program, Division of Medical Oncology/Hematology, University of Louisville School of Medicine, for his critical review and edition of this manuscript.
Author information
Authors and Affiliations
Contributions
C-YC, S-WL, M-MC, S-HH, Y-LL, J-RT, C-HT, C-NC, and Y-CC contributed to the conception and design of the study. C-YC, S-WL, M-MC, J-TK, S-HH, Y-LL, J-RT, C-HT, C-NC, and Y-CC contributed substantially to the data acquisition. C-YC, S-WL, J-TK, S-HH, Y-LL, J-RT, C-HT, and Y-CC contributed to statistical analysis and interpretation of data. All authors critically revised the manuscript and approved the submitted final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they had no interests which might be perceived as posing a conflict or bias.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Chang, CY., Lai, SW., Cheng, MM. et al. Real-world bleeding outcomes and product utilization in people with severe-type hemophilia A before and after switching to extended half-life rFVIIIFc prophylaxis therapy. Int J Hematol 117, 378–387 (2023). https://doi.org/10.1007/s12185-022-03503-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03503-9