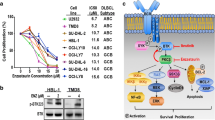
Abstract
Disease-risk stratification and development of intensified chemotherapy protocols have substantially improved the outcome of acute lymphoblastic leukemia (ALL). However, outcomes of relapsed or refractory cases remain poor. Previous studies have discussed the oncogenic role of enhancer of zeste homolog 1 and 2 (EZH1/2), and the efficacy of dual inhibition of EZH1/2 as a treatment for hematological malignancy. Here, we investigated whether an EZH1/2 dual inhibitor, DS-3201 (valemetostat), has antitumor effects on B cell ALL (B-ALL). DS-3201 inhibited growth of B-ALL cell lines more significantly and strongly than the EZH2-specific inhibitor EPZ-6438, and induced cell cycle arrest and apoptosis in vitro. RNA-seq analysis to determine the effect of DS-3201 on cell cycle arrest-related genes expressed by B-ALL cell lines showed that DS-3201 upregulated CDKN1C and TP53INP1. CRIPSR/Cas9 knockout confirmed that CDKN1C and TP53INP1 are direct targets of EZH1/2 and are responsible for the antitumor effects of DS-3201 against B-ALL. Furthermore, a patient-derived xenograft (PDX) mouse model showed that DS-3201 inhibited the growth of B-ALL harboring MLL-AF4 significantly. Thus, DS-3201 provides another option for treatment of B-ALL.





Similar content being viewed by others
References
Malard F, Mohty M. Acute lymphoblastic leukemia. Lancet. 2020;395:1146–62.
Li B, Chng WJ. EZH2 abnormalities in lymphoid malignancies: underlying mechanisms and therapeutic implications. J Hematol Oncol. 2019;12:118.
Chen J, Li J, Han Q, Sun Z, Wang J, Wang S, et al. Enhancer of zeste homolog 2 is overexpressed and contributes to epigenetic inactivation of p21 and phosphatase and tensin homolog in B-cell acute lymphoblastic leukemia. Exp Biol Med. 2012;237:1110–6.
Mochizuki-Kashio M, Aoyama K, Sashida G, Oshima M, Tomioka T, Muto T, et al. Ezh2 loss in hematopoietic stem cells predisposes mice to develop heterogeneous malignancies in an Ezh1-dependent manner. Blood. 2015;126:1172–83.
Neff T, Sinha AU, Kluk MJ, Zhu N, Khattab MH, Stein L, et al. Polycomb repressive complex 2 is required for MLL-AF9 leukemia. Proc Natl Acad Sci USA. 2012;109:5028–33.
Kim W, Bird GH, Neff T, Guo G, Kerenyi MA, Walensky LD, et al. Targeted disruption of the EZH2-EED complex inhibits EZH2-dependent cancer. Nat Chem Biol. 2013;9:643–50.
Honma D, Kanno O, Watanabe J, Kinoshita J, Hirasawa M, Nosaka E, et al. Novel orally bioavailable EZH1/2 dual inhibitors with greater antitumor efficacy than an EZH2 selective inhibitor. Cancer Sci. 2017;108:2069–78.
Fujita S, Honma D, Adachi N, Araki K, Takamatsu E, Katsumoto T, et al. Dual inhibition of EZH1/2 breaks the quiescence of leukemia stem cells in acute myeloid leukemia. Leukemia. 2018;32:855–64.
Nakagawa M, Fujita S, Katsumoto T, Yamagata K, Ogawara Y, Hattori A, et al. Dual inhibition of enhancer of zeste homolog 1/2 overactivates WNT signaling to deplete cancer stem cells in multiple myeloma. Cancer Sci. 2019;110:194–208.
Yamagishi M, Hori M, Fujikawa D, Ohsugi T, Honma D, Adachi N, et al. Targeting excessive EZH1 and EZH2 activities for abnormal histone methylation and transcription network in malignant lymphomas. Cell Rep. 2019;29:2321–37.
Kagiyama Y, Fujita S, Shima Y, Yamagata K, Katsumoto T, Nakagawa M, et al. CDKN1C-mediated growth inhibition by an EZH1/2 dual inhibitor overcomes resistance of mantle cell lymphoma to ibrutinib. Cancer Sci. 2021;112:2314–24.
Bruserud Ø, Glenjen N, Ryningen A, Ulvestad E. In vitro culture of human acute lymphoblastic leukemia (ALL) cells in serum-free media; a comparison of native ALL blasts, ALL cell lines and virus-transformed B cell lines. Leuk Res. 2003;27:455–64.
McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–12.
Qi W, Chan H, Teng L, Li L, Chuai S, Zhang R, et al. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci USA. 2012;109:21360–5.
Ntziachristos P, Tsirigos A, Van Vlierberghe P, Nedjic J, Trimarchi T, Flaherty MS, et al. Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat Med. 2012;18:298–301.
León TE, Rapoz-D’Silva T, Bertoli C, Rahman S, Magnussen M, Philip B, et al. EZH2-deficient T-cell acute lymphoblastic leukemia is sensitized to CHK1 inhibition through enhanced replication stress. Cancer Dsicov. 2020;10:998–1017.
Brown RE, Konopka KE, Weerasinghe P, Jaitly V, Dasgupta A, McGuire MF, et al. Morphoproteomics identifies SIRT1 and EZH2 pathways as commonalities in B-cell acute lymphoblastic leukemia: pathogenetic implications and opportunities for therapeutic intervention. Ann Clin Lab Sci. 2017;47:3–9.
Xu B, On DM, Ma A, Parton T, Konze KD, Pattenden SG, et al. Selective inhibition of EZH2 and EZH1 enzymatic activity by a small molecule suppresses MLL-rearranged leukemia. Blood. 2015;125:346–57.
Li H, Kong X, Cui G, Ren C, Fan S, Sun L, et al. Rapamycin restores p14, p15 and p57 expression and inhibits the mTOR/p70S6K pathway in acute lymphoblastic leukemia cells. Int J Hematol. 2015;102:558–68.
Zhang W, Kuang P, Liu T. Prognostic significance of CDKN2A/B deletions in acute lymphoblastic leukaemia: a meta-analysis. Ann Med. 2019;51:28–40.
Ragusa D, Makarov EM, Britten O, Moralli D, Green CM, Tosi S. The RS4;11 cell line as a model for leukaemia with t(4;11)(q21;q23): Revised characterisation of cytogenetic features. Cancer Rep. 2019;2: e1207.
Shahbazi J, Lock R, Liu T. Tumor protein 53-induced nuclear protein 1 enhances p53 function and represses tumorigenesis. Front Genet. 2013;4:80.
Tomasini R, Seux M, Nowak J, Bontemps C, Carrier A, Dagorn JC, et al. TP53INP1 is a novel p73 target gene that induces cell cycle arrest and cell death by modulating p73 transcriptional activity. Oncogene. 2005;24:8093–104.
Ye W, Liang F, Ying C, Zhang M, Feng D, Jiang X, et al. Downregulation of microRNA-3934-5p induces apoptosis and inhibits the proliferation of neuroblastoma cells by targeting TP53INP1. Exp Ther Med. 2019;18:3729–36.
Demir S, Boldrin E, Sun Q, Hampp S, Tausch E, Eckert C, et al. Therapeutic targeting of mutant p53 in pediatric acute lymphoblastic leukemia. Haematologica. 2020;105:170–81.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
Perica K, Flynn J, Curran KJ, Rivere I, Wang X, Senechal B, et al. Impact of bridging chemotherapy on clinical outcome of CD19 CAR T therapy in adult acute lymphoblastic leukemia. Leukemia. 2021;35:3268–71.
Acknowledgements
The authors would like to thank Daisuke Honma (Daiichi Sankyo, Co., Ltd.) for providing DS-3201. This work was supported by the Project for Cancer Research and Therapeutic Evolution (P-CREATE).
Author information
Authors and Affiliations
Contributions
JI and KY designed the study and conducted the experiments. HS, YS, TK, and YA provided technical support for experiments. JI prepared the figures and wrote the manuscript. IK supervised the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12185_2022_3469_MOESM1_ESM.eps
Supplementary Figure 1. Flow cytometry analysis of the cell cycle and apoptosis in KP-L-RY cells. (A) Representative flow cytometry plots showing the cell cycle (left) and apoptosis (right) profiles of KP-L-RY cells. (B) Bar graph showing the mean percentage of cells ± SD at each cell cycle phase (left) and the percentage of Annexin V positive cells (right). Cells were treated for 10 days with vehicle alone or with 0.5 μmol /L DS-3201. Data represent the mean (of triplicates) ± s.d; Supplementary Figure 2. Dual knockout of CDKN1C and TP53INP1 in BALL-1 cells. (A) Western blotting of CDKN1C and TP53INP1 in Cas9-expressing BALL-1 cells harboring nontargeting sgRNA or both sgCDKN1C #1 + sgTP53INP1 #1. Lysates were obtained from cells exposed for 7 days to vehicle alone or 0.5 μmol/L DS-3201. β-actin was used as a loading control. (B) Growth curves of CDKN1C and/or TP53INP1 knockout BALL-1 cells treated for 10 days with vehicle alone or with 0.5 μmol/L DS-3201. Data represent the mean (of triplicates) ± s.d. (EPS 959 KB)
About this article
Cite this article
Ito, J., Yamagata, K., Shinohara, H. et al. Dual inhibition of EZH1/2 induces cell cycle arrest of B cell acute lymphoblastic leukemia cells through upregulation of CDKN1C and TP53INP1. Int J Hematol 117, 78–89 (2023). https://doi.org/10.1007/s12185-022-03469-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03469-8