Abstract
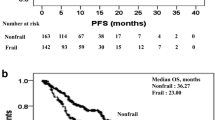
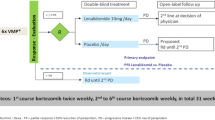
We conducted a post hoc analysis of our previous pilot observational study on the efficacy and safety of carfilzomib (CFZ)-containing therapy in 50 patients with relapsed/refractory multiple myeloma in routine practice to clarify the relationships between three major criteria for vulnerability (frailty, poor performance status [PS], and advanced age [≥ 75 years]) and their clinical impact on efficacy and adverse events (AEs). Sixteen patients fulfilled at least one and five patients fulfilled all three criteria. The overall response rate was not significantly affected by frailty, poor PS, and/or advanced age; however, frailty and advanced age were significantly associated with shorter progression-free survival (PFS). In contrast, no significant difference in PFS was observed between patients with PS0–1 or PS2–4. The three criteria for vulnerability were associated with more frequent hematologic AEs: frailty, poor PS, and/or advanced age significantly increased the risk of grade 3–4 anemia and lymphopenia. However, these criteria were not associated with increased risk of other non-hematologic AEs except infection. Collectively, these results demonstrate the need to carefully manage severe hematologic AEs in vulnerable patients and perform disease-specific assessment of frailty to predict prognosis.

Similar content being viewed by others
References
Pulte D, Gondos A, Brenner H. Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. Oncologist. 2011;16(11):1600–3.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–52.
Siegel DS, Dimopoulos MA, Ludwig H, Facon T, Goldschmidt H, Jakubowiak A, et al. Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36(8):728–34.
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38.
Dimopoulos MA, Goldschmidt H, Niesvizky R, Joshua D, Chng W-J, Oriol A, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:1327–37.
Kawaji-Kanayama Y, Kobayashi T, Muramatsu A, Uchiyama H, Sasaki N, Uoshima N, et al. Prognostic impact of resistance to bortezomib and/or lenalidomide in carfilzomib-based therapies for relapsed/refractory multiple myeloma: the Kyoto Clinical Hematology Study Group, multicenter, pilot, prospective, observational study in Asian patients. Cancer Rep. 2021;e1476.
Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125(13):2068–74.
Richardson PG, San Miguel JF, Moreau P, Hajek R, Dimopoulos MA, Laubach JP, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8(11):109.
Patel BG, Luo S, Wildes TM, Sanfilippo KM. Frailty in older adults with multiple myeloma: a study of US veterans. JCO Clin Cancer Inform. 2020;4:117–27.
Bang SM, Kyle RA, Rajkumar SV, Kumar S. Treatment patterns and outcomes in elderly patients with multiple myeloma. Leukemia. 2013;27(4):971–4.
Kint N, Delforge M. Concise review—treatment of multiple myeloma in the very elderly: how do novel agents fit in? J Geriatr Oncol. 2016;7(5):383–9.
Wildes TM, Anderson KC. Approach to the treatment of the older, unfit patient with myeloma from diagnosis to relapse: perspectives of a US hematologist and a geriatric hematologist. Hematol Am Soc Hematol Educ Program. 2018;2018(1):88–96.
Antoine-Pepeljugoski C, Braunstein MJ. Management of newly diagnosed elderly multiple myeloma patients. Curr Oncol Rep. 2019;21(7):64.
Facon T, Niesvizky R, Mateos MV, Siegel D, Rosenbaum C, Bringhen S, et al. Efficacy and safety of carfilzomib-based regimens in frail patients with relapsed and/or refractory multiple myeloma. Blood Adv. 2020;4(21):5449–59.
Chari A, Romanus D, Palumbo A, Blazer M, Farrelly E, Raju A, et al. Randomized clinical trial representativeness and outcomes in real-world patients: comparison of 6 hallmark randomized clinical trials of relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2020;20(1):8-17.e16.
Ludwig H, Dimopoulos MA, Moreau P, Chng WJ, Goldschmidt H, Hájek R, et al. Carfilzomib and dexamethasone vs bortezomib and dexamethasone in patients with relapsed multiple myeloma: results of the phase 3 study ENDEAVOR (NCT01568866) according to age subgroup. Leuk Lymphoma. 2017;58(10):2501–4.
Davies F, Rifkin R, Costello C, Morgan G, Usmani S, Abonour R, et al. Real-world comparative effectiveness of triplets containing bortezomib (B), carfilzomib (C), daratumumab (D), or ixazomib (I) in relapsed/refractory multiple myeloma (RRMM) in the US. Ann Hematol. 2021;100(9):2325–37.
Chari A, Richardson PG, Romanus D, Dimopoulos MA, Sonneveld P, Terpos E, et al. Real-world outcomes and factors impacting treatment choice in relapsed and/or refractory multiple myeloma (RRMM): a comparison of VRd, KRd, and IRd. Expert Rev Hematol. 2020;13(4):421–33.
Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–60.
Isaac S, Michael WB. Handbook in research and evaluation: a collection of principles, methods, and strategies useful in planning, design, and evaluation of studies in education and the behavioral sciences. 3rd ed. San Diego: Educational and Industrial Testing Services; 1997.
Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Stat. 2005;4:287–91.
Connelly LM. Pilot studies. Medsurg Nurs. 2008;17(6):411–2.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–55.
Engelhardt M, Dold SM, Ihorst G, Zober A, Möller M, Reinhardt H, et al. Geriatric assessment in multiple myeloma patients: validation of the International Myeloma Working Group (IMWG) score and comparison with other common comorbidity scores. Haematologica. 2016;101(9):1110–9.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48(3):452–8.
Bringhen S, Mateos MV, Zweegman S, Larocca A, Falcone AP, Oriol A, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013;98(6):980–7.
Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–65.
Palmieri S, Rocco S, Vitagliano O, Catalano L, Cerchione C, Vincelli ID, et al. KRD (carfilzomib and lenalidomide plus dexamethasone) for the treatment of relapsed or refractory multiple myeloma in the real-life: a retrospective survey in 123 patients. Ann Hematol. 2020;99(12):2903–9.
Rocchi S, Tacchetti P, Pantani L, Mancuso K, Rizzello I, di Giovanni BC, et al. A real-world efficacy and safety analysis of combined carfilzomib, lenalidomide, and dexamethasone (KRd) in relapsed/refractory multiple myeloma. Hematol Oncol. 2021;39(1):41–50.
Mele A, Prete E, De Risi C, Citiso S, Greco G, Falcone AP, et al. Carfilzomib, lenalidomide, and dexamethasone in relapsed/refractory multiple myeloma patients: the real-life experience of Rete Ematologica Pugliese (REP). Ann Hematol. 2021;100(2):429–36.
Keats JJ, Chesi M, Egan JB, Garbitt VM, Palmer SE, Braggio E, et al. Clonal competition with alternating dominance in multiple myeloma. Blood. 2012;120(5):1067–76.
Schinke M, Ihorst G, Duyster J, Wäsch R, Schumacher M, Engelhardt M. Risk of disease recurrence and survival in patients with multiple myeloma: a German Study Group analysis using a conditional survival approach with long-term follow-up of 815 patients. Cancer. 2020;126(15):3504–15.
Mitra AK, Harding T, Mukherjee UK, Jang JS, Li Y, Hong Zheng R, et al. A gene expression signature distinguishes innate response and resistance to proteasome inhibitors in multiple myeloma. Blood Cancer J. 2017;7(6):e581.
Brünnert D, Kraus M, Stühmer T, Kirner S, Heiden R, Goyal P, et al. Novel cell line models to study mechanisms and overcoming strategies of proteasome inhibitor resistance in multiple myeloma. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1666–76.
Soriano GP, Besse L, Li N, Kraus M, Besse A, Meeuwenoord N, et al. Proteasome inhibitor-adapted myeloma cells are largely independent from proteasome activity and show complex proteomic changes, in particular in redox and energy metabolism. Leukemia. 2016;30(11):2198–207.
Dimopoulos M, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186–97.
Quach H, Nooka A, Samoylova O, Venner CP, Kim K, Facon T, et al. Carfilzomib, dexamethasone and daratumumab in relapsed or refractory multiple myeloma: results of the phase III study CANDOR by prior lines of therapy. Br J Haematol. 2021;194(4):784–8.
Scheubeck S, Ihorst G, Schoeller K, Holler M, Möller MD, Reinhardt H, et al. Comparison of the prognostic significance of 5 comorbidity scores and 12 functional tests in a prospective multiple myeloma patient cohort. Cancer. 2021;127(18):3422–36.
Kumar S, Fu A, Niesvizky R, Jagannath S, Boccia R, Raje N. Renal response in real-world carfilzomib- vs bortezomib-treated patients with relapsed or refractory multiple myeloma. Blood Adv. 2021;5(2):367–76.
Dimopoulos M, Siegel D, White DJ, Boccia R, Iskander KS, Yang Z, et al. Carfilzomib vs bortezomib in patients with multiple myeloma and renal failure: a subgroup analysis of ENDEAVOR. Blood. 2019;133(2):147–55.
Dimopoulos MA, Stewart AK, Masszi T, Špička I, Oriol A, Hájek R, et al. Carfilzomib, lenalidomide, and dexamethasone in patients with relapsed multiple myeloma categorised by age: secondary analysis from the phase 3 ASPIRE study. Br J Haematol. 2017;177(3):404–13.
Orlowski RZ, Moreau P, Niesvizky R, Ludwig H, Oriol A, Chng WJ, et al. Carfilzomib-dexamethasone versus bortezomib-dexamethasone in relapsed or refractory multiple myeloma: updated overall survival, safety, and subgroups. Clin Lymphoma Myeloma Leuk. 2019;19(8):522–30.
Weisel K, Mateos MV, Gay F, Delforge M, Cook G, Szabo Z, et al. Efficacy and safety profile of deep responders to carfilzomib-based therapy: a subgroup analysis from ASPIRE and ENDEAVOR. Leukemia. 2021;35(6):1732–44.
Conticello C, Romano A, Del Fabro V, Martino EA, Calafiore V, Sapienza G, et al. Feasibility, tolerability and efficacy of carfilzomib in combination with lenalidomide and dexamethasone in relapsed refractory myeloma patients: a retrospective real-life survey of the Sicilian Myeloma Network. J Clin Med. 2019;8(6):877.
Acknowledgements
We thank all the researchers in the Kyoto Clinical Hematology Study Group for their scientific support. This work was funded by Ono Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Consortia
Contributions
YK, AM, TK, and JK analyzed and interpreted the data. JK and TK were involved in the study conception and design. YK, AM, NS, KS, MK, SF, RI, TF, YM, TT, YC, SM, MN, HK, EK, KH, RT, CS, HU, NO, and YS were involved in data acquisition. TK and YK performed statistical analysis. YK drafted the manuscript. JK revised the manuscript. MT and JK supervised the research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
This study is supported by Ono Pharmaceutical. J.K. has received research funding from Bristol-Myers Squibb, Sysmex, Ono Pharmaceutical, Sanofi, Takeda, Fujimoto Pharmaceutical, and Abbvie; has received honoraria from Bristol-Myers Squibb, Janssen Pharmaceutical K.K, Ono Pharmaceutical, Takeda, Sanofi, Fujimoto Pharmaceutical, and Abbvie; and is a consultant for Janssen Pharmaceutical K.K, and Bristol-Myers Squibb. T.K. has received honoraria from Ono Pharmaceutical. M.T. has received honoraria from Bristol-Myers Squibb. N.U. has received honoraria from Bristol-Myers Squibb and Takeda. S.F. has received honoraria from Takeda, Ono Pharmaceutical, Bristol-Myers Squibb, Sanofi, and Janssen Pharmaceutical. All other authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kawaji-Kanayama, Y., Muramatsu, A., Sasaki, N. et al. Clinical impacts of frailty, poor performance status, and advanced age in carfilzomib-containing treatment for relapsed/refractory multiple myeloma: post hoc investigation of the KOTOSG multicenter pilot prospective observational study. Int J Hematol 115, 350–362 (2022). https://doi.org/10.1007/s12185-021-03262-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03262-z