Abstract
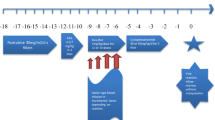
A 43-year-old Japanese male, who had undergone open liver surgery for tumor resection, presented with decreased hemoglobin levels on Day 13 post-emergency-release transfusion of 16 units of Fy(a +) red blood cells. As the anemia was accompanied by increased lactate dehydrogenase, indirect bilirubin, and reticulocytes, as well as decreased haptoglobin, it was attributed to hemolysis. In the diagnostic workup for hemolytic reaction, the direct antiglobulin test result for IgG was positive and the antibody dissociated from the patient’s peripheral red blood cells was identified as anti-Fya (titer, 4). The hemolytic reaction was transient (approximately 10 days), of moderate severity, and did not result in any obvious organ damage. However, a single compatible red blood cell transfusion of 2 units was required on Day 17 after the causative transfusion. Notably, HLA typing revealed that the patient carried the HLA-DRB1*04:03 allele, which has been implicated in immunogenicity and induction of anti-Fya response in Caucasian populations. In summary, this is the first documented case of definitive anti-Fya-mediated delayed hemolytic transfusion reaction associated with HLA-DRB1*04:03 in the Japanese population.



Similar content being viewed by others
References
Maeda H, Ohto H, Okazaki H. Transfusion medicine. 4th ed. Tokyo: Chugai-Igakusha; 2018.
Moore SB, Taswell HF, Pineda AA, Sonnenberg CL. Delayed hemolytic transfusion reactions. Evidence of the need for an improved pretransfusion compatibility test. Am J Clin Pathol. 1980;74:94–7.
Sawabe T, Miya T, Yashiro T, Takayama J, Tobinai K, Takenaka T. Delayed hemolytic transfusion reaction during past ten years. Jpn J Trans Med. 1993;39:974–8.
2016 Nationwide Survey on Adverse Events in Blood Transfusion in Japan. [cited Jul 5, 2021]. Available from: http://yuketsu.jstmct.or.jp/wp-content/uploads/2018/06/d1cac760f68752cfab338a81d461bf57.pdf [in Japanese]
2018 Nationwide Survey on Adverse Events in Blood Transfusion in Japan. [cited Jul 5, 2021]. Available from: http://yuketsu.jstmct.or.jp/wp-content/uploads/2020/10/0ead9f9f7c07dc2078c3975297d0feb2.pdf [in Japanese]
Milkins C, Knowles S, DeSilva M, Contreras M, Stainsby D. Prevention and diagnosis of delayed haemolytic transfusion reactions. Vox Sang. 2006;91:365–7.
Cutbush M, Mollison PL, Parkin DM. A new human blood group. Nature. 1950;165:188–9.
Ikin EW, Mourant AE, Pettenkofer JH, Blumenthal G. Discovery of the expected haemagglutinin, anti-Fyb. Nature. 1951;168:1077–8.
Pogo AO, Chaudhuri A. The Duffy protein: a malarial and chemokine receptor. Semin Hematol. 2000;37:122–9.
Klein HG, Anstee DJ. Mollison’s blood transfusion in clinical medicine. 12the ed. Oxford: Blackwell; 2014.
Guidelines for Action Against Intraoperative Critical Hemorrhage. [cited Jul 30, 2021]. Available from: http://yuketsu.jstmct.or.jp/wp-content/themes/jstmct/images/medical/file/guidelines/Ref4-1.pdf [in Japanese]
Itoh Y, Mizuki N, Shimada T, Azuma F, Itakura M, Kashiwase K, et al. High-throughput DNA typing of HLA-A, -B, -C, and -DRB1 loci by a PCR-SSOP-Luminex method in the Japanese population. Immunogenetics. 2005;57:717–29.
Reynisson B, Barra C, Kaabinejadian S, Hildebrand WH, Peters B, Nielsen M. Improved prediction of MHC II antigen presentation through integration and motif deconvolution of mass spectrometry MHC eluted ligand data. J Proteome Res. 2020;19:2304–15.
Giblet ER. A critique of the theorical hazard of inter vs intra-racial transfusion. Transfusion. 1961;1:233–8.
Stott LM, Barker RN, Urbaniak SJ. Identification of alloreactive T-cell epitopes on the Rhesus D protein. Blood. 2000;96:4011–9.
Ansart-Pirenne H, Zeliszewski D, Lee K, Martin-Blanc S, Rouger P, Noizat-Pirenne F. Identification of immunodominant alloreactive T-cell epitopes on the Jka red blood cell protein inducing either Th1 or Th2 cytokine expression. Blood. 2004;104:3409–10.
Noizat-Pirenne F, Tournamille C, Bierling P, Roudot-Thoraval F, Le Pennec PY, Rouger P, et al. Relative immunogenicity of Fya and K antigens in a Caucasian population, based on HLA class II restriction analysis. Transfusion. 2006;46:1328–33.
Picard C, Frassati C, Basire A, Buhler S, Galicher V, Ferrera V, et al. Positive association of DRB1*04 and DRB1*15 alleles with Fya immunization in a Southern European population. Transfusion. 2009;49:2412–7.
Schonewille H, Doxiadis I, Levering W, Roelen D, Claas F, Brand A. HLA-DRB1 associations in individuals with single and multiple clinically relevant red blood cell antibodies. Transfusion. 2014;54:1971–80.
HLA haplotype/allele frequency. [Cited Jul 30, 2021]. Available from: https://hla.or.jp/med/frequency_search/en/sero/
Wada H, Takahashi H, Uchiyama T, Eguchi Y, Okamoto K, Kawasugi K, Madoiwa S, et al. The approval of revised diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J. 2017;15:17.
Acknowledgements
The authors thank Shoko Arakawa, Shota Oikawa, Yuto Sakamoto, Yume Tomiya, Shota Fujiki, Tomoki Yada, Takumi Negishi, and Hayato Kojima for their contributions to this work.
Funding
This work was supported in part by Grants-in-Aid from the Fujita Health University Research Grant Program (YM and SF) and by JSPS KAKENHI Grant Number 21K15655 (HM).
Author information
Authors and Affiliations
Contributions
TM and YS: Conception and study design, collection of study materials, data collection, analysis and interpretation, and manuscript writing. HM: conception and study design, collection of study materials, data collection, analysis and interpretation, financial support, and manuscript writing. RS: collection of study materials, data collection, analysis and interpretation, and manuscript writing. SF and YM: conception and study design, data analysis and interpretation, financial support, and manuscript writing. All authors approved the final version of the manuscript and agreed to all aspects of the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Matsuno, T., Matsuura, H., Fujii, S. et al. Anti-Fya-mediated delayed hemolytic transfusion reaction following emergency-release red blood cell transfusion: possible involvement of HLA-DRB1*04:03 in the Japanese population. Int J Hematol 115, 440–445 (2022). https://doi.org/10.1007/s12185-021-03242-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03242-3