Abstract
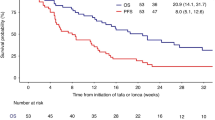
Patients with high-risk diffuse large B-cell lymphoma (DLBCL) have poor outcomes following first-line cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab (R-CHOP). Evidence shows chemotherapy and immune checkpoint blockade can increase antitumor efficacy. This study investigated durvalumab, a programmed death-ligand 1 inhibitor, combined with R-CHOP or lenalidomide + R-CHOP (R2-CHOP) in newly diagnosed high-risk DLBCL. Patients received durvalumab 1125 mg every 21 days for 2–8 cycles + R-CHOP (non-activated B-cell [ABC] subtype) or R2-CHOP (ABC), then durvalumab consolidation (1500 mg every 28 days). Of 46 patients, 43 received R-CHOP and three R2-CHOP. All patients had the high-risk disease; 14 (30.4%) and eight (17.4%) had double- or triple-hit DLBCL, respectively. Following induction, 20/37 (54.1%) patients receiving durvalumab + R-CHOP achieved complete response (CR), and seven (18.9%) partial response (PR); 25 (67.6% [95% CI 50.2–82.0]) continued to consolidation and were progression-free at 12 months. Among efficacy-evaluable patients with double- or triple-hit DLBCL (n = 12), five achieved CR and five PR. Adverse events were generally consistent with R-CHOP. Correlative analyses did not identify conclusive biomarkers of response. Durvalumab + R-CHOP is feasible in DLBCL with no new safety signals, but the combination provided no greater benefit than R-CHOP.




Similar content being viewed by others
Data sharing statement
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11.
Fu K, Weisenburger DD, Choi WW, Perry KD, Smith LM, Shi X, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol. 2008;26:4587–94.
Nowakowski GS, LaPlant B, Macon WR, Reeder CB, Foran JM, Nelson GD, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol. 2015;33:251–7.
Vitolo U, Witzig TE, Gascoyne RD, Scott DW, Zhang Q, Jurczak W, et al. ROBUST: first report of phase III randomized study of lenalidomide/R-CHOP (R2-CHOP) versus placebo/R-CHOP in previously untreated ABC-type diffuse large B-cell lymphoma [abstract]. Hematol Oncol. 2019;37:36–7.
Nowakowski GS, Hong F, Scott DW, Macon R, King RL, Habermann TM, et al. Addition of lenalidomide to R-CHOP (R2CHOP) improves outcomes in newly diagnosed diffuse large B-cell lymphoma (DLBCL): first report of ECOG-ACRIN1412 a randomized phase 2 US intergroup study of R2CHOP versus R-CHOP [abstract]. Hematol Oncol. 2019;37:37–8.
Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. 2017;14:203–20.
Kiyasu J, Miyoshi H, Hirata A, Arakawa F, Ichikawa A, Niino D, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126:2193–201.
Rossille D, Azzaoui I, Feldman AL, Maurer MJ, Laboure G, Parrens M, et al. Soluble programmed death-ligand 1 as a prognostic biomarker for overall survival in patients with diffuse large B-cell lymphoma: a replication study and combined analysis of 508 patients. Leukemia. 2017;31:988–91.
Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. 2008;45:1470–6.
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95.
Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–33.
Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–701.
Oganesyan V, Gao C, Shirinian L, Wu H, Dall’Acqua WF. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr. 2008;64:700–4.
Ibrahim R, Stewart R, Shalabi A. PD-L1 blockade for cancer treatment: MEDI4736. Semin Oncol. 2015;42:474–83.
Yang H, Shen K, Zhu C, Li Q, Zhao Y, Ma X. Safety and efficacy of durvalumab (MEDI4736) in various solid tumors. Drug Des Dev Ther. 2018;12:2085–96.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90.
Scott DW, Wright GW, Williams PM, Lih CJ, Walsh W, Jaffe ES, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood. 2014;123:1214–7.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Higgs BW, Morehouse CA, Streicher K, Brohawn PZ, Pilataxi F, Gupta A, et al. Interferon gamma messenger RNA signature in tumor biopsies predicts outcomes in patients with non-small cell lung carcinoma or urothelial cancer treated with durvalumab. Clin Cancer Res. 2018;24:3857–66.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Younes A, Burke JM, Cheson BD, Diefenbach C, Ferrari S, Hahn UH, et al. Safety and efficacy of atezolizumab in combination with rituximab plus CHOP in previously untreated patients with diffuse large B-cell lymphoma (DLBCL): updated analysis of a phase I/II study [abstract]. Blood. 2019;134(suppl 1):2874.
Younes A, Burke JM, Cheson B, Diefenbach C, Ferrari S, Hahn U, et al. Safety and efficacy of atezolizumab in combination with rituximab plus CHOP in previously untreated patients with diffuse large B-cell lymphoma (DLBCL): primary analysis of a phase I/II study [abstract]. Blood Adv. 2018;132:2969.
Smith SD, Till BG, Shadman MS, Lynch RC, Cowan AJ, Wu QV, et al. Pembrolizumab with R-CHOP in previously untreated diffuse large B-cell lymphoma: potential for biomarker driven therapy. Br J Haematol. 2020;189:1119–26.
Hawkes EA, Chong G, Smith C, Lee S-T, Churilov L, McKendrick J, et al. Safety and efficacy of induction and maintenance avelumab plus R-CHOP in patients with diffuse large B-cell lymphoma (DLBCL): analysis of the phase II Avr-CHOP study [abstract 597]. Blood. 2020;136:43–4.
Ansell SM, Minnema MC, Johnson P, Timmerman JM, Armand P, Shipp MA, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, phase II study. J Clin Oncol. 2019;37:481–9.
Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34:2698–704.
Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, Fernando D, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15:6446–53.
Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–84.
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42.
Nowakowski GS, Hong F, Scott DW, Macon WR, King RL, Habermann TM, et al. Addition of lenalidomide to R-CHOP improves outcomes in newly diagnosed diffuse large B-cell lymphoma in a randomized phase II US Intergroup Study ECOG-ACRIN E1412. J Clin Oncol. 2021;39:J2001375.
Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460–7.
Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–9.
Acknowledgements
The authors thank the patients enrolled in the study as well as associated clinical trial teams. The authors received editorial support in the preparation of this manuscript from Lauren Gallagher, RPh, PhD, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, sponsored by Celgene, a Bristol Myers Squibb Company, Princeton, NJ, USA. The authors, however, directed and are fully responsible for all content and editorial decisions for this manuscript.
Funding
This trial (NCT03003520) was sponsored by Celgene, a Bristol Myers Squibb company, and supported by AstraZeneca/MedImmune.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
GSN has received grants from Celgene/Bristol Myers Squibb, Curis, Incyte, MorphoSys, Roche, and Seattle Genetics, and personal fees from Debiopharm, Karyopahrm, Kite, Kymera, Selvita, and TG Therapeutics. WW has served on steering and safety committees for Amgen, Celgene, DSMM, and Morphosys; was employed by Oncotyrol (20%); has served on advisory boards and as a consultant for Amgen, Bristol Myers Squibb/Celgene, Gilead, GSK, Incyte, Janssen, Novartis, Merck, Pfizer, Roche, Sandoz, Sanofi, and Takeda; has presented lectures for Amgen, AbbVie, Bristol Myers Squibb/Celgene, Fujimoto, Gilead, GSK, Janssen, Myelom- und Lymphomselbsthilfe Österreich, Novartis, Pfizer, Roche, Sandoz, Sanofi, and Takeda; and has received research funding from Amgen, Bristol Myers Squibb, Celgene, Janssen, Novartis, Roche, Sanofi, Takeda, oncotyrol, European Commission (FP7—OPTATIO), and Bundesland Tirol Programm “Translational research.” RG has received honoraria, consulting/advisory fees, research funding, and travel fees, accommodations, and/or expenses from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Merck, MSD, Novartis, Roche, Sandoz, and Takeda. TSL has served as a consultant and on advisory boards for Bristol Myers Squibb/Celgene, Novartis, Roche, and Gilead. KP has received grants and/or personal fees from AstraZeneca, Bristol Myers Squibb, Genentech, Kite Pharmaceuticals, Pharmacyclics/Janssen, and TG Therapeutics. UJ has served on the advisory board for Bristol Myers Squibb/Celgene, Roche, Novartis, and Janssen; and has served as a consultant for Miltenyi, Takeda, Merck, Incyte, and AbbVie. RFM, LT, and HE have nothing to disclose. NK has received research funding from Celgene and Roche UK, and has served on the advisory board for Bristol Myers Squibb, Gilead, Karyopharm, and Takeda. PB has served on the advisory board for Celgene, Incyte, Takeda, and Roche. JMJ has received personal fees from Bristol Myers Squibb/Celgene, Gilead/Kite Pharma, Novartis, and Roche. DC has received research funding from Amgen, AstraZeneca, Bayer, Celgene, Clovis, Eli Lilly, Janssen, MedImmune, Merck, Merrimack, Sanofi, and 4SC. BF, NDR, NK, and M-LC are employees of and stockholders in Bristol Myers Squibb. JD, NK, and OM were employees of and stockholders in Bristol Myers Squibb at the time of the study. JM has served as a consultant for Pharmacyclics, Bayer, Gilead/Kite Pharma, Pfizer, Janssen, Juno/Celgene, Bristol Myers Squibb, Kyowa, Alexion, Beigene, Fosunkite, Innovent, Seattle Genetics, and Beigene; has received research funding from Bayer, Gilead/Kite Pharma, Celgene, Merck, Portola, Incyte, Genentech, Pharmacyclics, Seattle Genetics, Janssen, and Millennium; has received honoraria from Kyowa and Seattle Genetics; and has served on the speakers bureau for Gilead/Kite Pharma, Kyowa, Bayer, Pharmacyclics/Janssen, Seattle Genetics, Acrotech/Aurobindo, Beigene, Verastem, AstraZeneca, Celgene/Bristol Myers Squibb, Genentech/Roche, and AbbVie.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Justine Dell’Aringa, Nurgul Kilavuz and Oliver Manzke: At the time of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Nowakowski, G.S., Willenbacher, W., Greil, R. et al. Safety and efficacy of durvalumab with R-CHOP or R2-CHOP in untreated, high-risk DLBCL: a phase 2, open-label trial. Int J Hematol 115, 222–232 (2022). https://doi.org/10.1007/s12185-021-03241-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03241-4