Abstract
A combination of three post-transplant drugs, cyclophosphamide (PTCy), a calcineurin inhibitor, and mycophenolate mofetil, has long been used for prophylaxis of graft-versus-host-disease (GVHD) after HLA-haploidentical allogeneic hematopoietic cell transplantation (allo-HCT). Recently, this combination has been used following HLA-matched allo-HCT as well, but the optimal combination of drugs for GVHD prophylaxis in an HLA-matched setting remains unclear. This prospective phase II study evaluated the safety and efficacy of PTCy plus tacrolimus (TAC) for GVHD prophylaxis after allo-HCT from HLA-matched related donors (MRD) or HLA-matched unrelated donors (MUD). The cumulative incidences of grades II–IV and III–IV acute GVHD at 100 days post-transplantation were 18% and 5.9%, respectively, in the MRD group, and 18% and 9.1%, respectively, in the MUD group. The cumulative incidences of moderate to severe chronic GVHD at 1 year were 12% and 9.1% in the MRD and MUD groups, respectively. The 1-year overall survival rates in the MRD and MUD groups were 88% and 64%, respectively, and the 1-year GVHD-free, relapse free survival rates were 59% and 50%, respectively. These results suggest that GVHD prophylaxis with a less intensive double drug combination (PT/Cy and TAC) might be feasible after HLA-matched allo-HCT.
Clinical Trial Notation This trial was a prospective single-center trial registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR; identification number: UMIN000023890) and the Japan Registry of Clinical Trials (jRCTs051180143).



Similar content being viewed by others
Change history
01 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12185-021-03252-1
References
Lee CJ, Savani BN, Mohty M, Labopin M, Ruggeri A, Schmid C, et al. Haploidentical hematopoietic cell transplantation for adult acute myeloid leukemia: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102:1810–22.
D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26:e177–82.
Gu Z, Wang L, Yuan L, Huang W, Li M, Guan L, et al. Similar outcomes after haploidentical transplantation with post-transplant cyclophosphamide versus HLA-matched transplantation: a meta-analysis of case-control studies. Oncotarget. 2017;8:63574–86.
Meybodi MA, Cao W, Luznik L, Bashey A, Zhang X, Romee R, et al. HLA-haploidentical vs matched-sibling hematopoietic cell transplantation: a systematic review and meta-analysis. Blood Adv. 2019;3:2581–5.
Gagelmann N, Bacigalupo A, Rambaldi A, Hoelzer D, Halter J, Sanz J, et al. Haploidentical stem cell transplantation with posttransplant cyclophosphamide therapy vs other donor transplantations in adults with hematologic cancers: a systematic review and meta-analysis. JAMA Oncol. 2019;5:1739–48.
Arcuri LJ, Mesquita Aguiar MT, Freitoso Ribeiro AA, Fonseca Pacheco AG. Haploidentical transplantation with post-transplant cyclophosphamide versus unrelated donor hematopoietic stem cell transplantation: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2019;25:2422–30.
Bradstock KF, Bilmon I, Kwan J, Micklethwaite K, Blyth E, Deren S, et al. Single-agent high-dose cyclophosphamide for graft-versus-host disease prophylaxis in human leukocyte antigen-matched reduced-intensity peripheral blood stem cell transplantation results in an unacceptably high rate of severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:941–4.
Holtick U, Chemnitz JM, Shimabukuro-Vornhagen A, Theurich S, Chakupurakal G, Krause A, et al. OCTET-CY: a phase II study to investigate the efficacy of post-transplant cyclophosphamide as sole graft-versus-host prophylaxis after allogeneic peripheral blood stem cell transplantation. Eur J Haematol. 2016;96:27–35.
Moiseev IS, Pirogova OV, Alyanski AL, Babenko EV, Gindina TL, Darskaya EI, et al. Graft-versus-host disease prophylaxis in unrelated peripheral blood stem cell transplantation with post transplantation cyclophosphamide, tacrolimus, and mycophenolate mofetil. Biol Blood Marrow Transplant. 2016;22:1037–42.
Shah MV, Saliba RM, Rondon G, Chen J, Soebbing D, Rus I, et al. Pilot study using post-transplant cyclophosphamide (PTCy), tacrolimus and mycophenolate GVHD prophylaxis for older patients receiving 10/10 HLA-matched unrelated donor hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019;54:601–6.
Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6:e132–43.
Carnevale-Schianca F, Caravelli D, Gallo S, Coha V, D’Ambrosio L, Vassallo E, et al. Post-transplant cyclophosphamide and tacrolimus-mycophenolate mofetil combination prevents graft-versus-host disease in allogeneic peripheral blood hematopoietic cell transplantation from HLA-matched donors. Biol Blood Marrow Transplant. 2017;23:459–66.
Mielcarek M, Furlong T, O’Donnell PV, Storer BE, McCune JS, Storb R, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–8.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–8.
Nakamae H, Koh H, Katayama T, Nishimoto M, Hayashi Y, Nakashima Y, et al. HLA haploidentical peripheral blood stem cell transplantation using reduced dose of posttransplantation cyclophosphamide for poor-prognosis or refractory leukemia and myelodysplastic syndrome. Exp Hematol. 2015;43:921–9.
Sugita J, Kawashima N, Fujisaki T, Kakihana K, Ota S, Matsuo K, et al. Japan Study Group for Cell Therapy and Transplantation HLA-haploidentical peripheral blood stem cell transplantation with post-transplant cyclophosphamide after busulfan-containing reduced-intensity conditioning. Biol Blood Marrow Transplant. 2015;21:1646–52.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Nakamae H, Fujii K, Nanno S, Okamura H, Nakane T, Koh H, et al. A prospective observational study of immune reconstitution following transplantation with post-transplant reduced-dose cyclophosphamide from HLA-haploidentical donors. Transpl Int. 2019;32:1322–32.
Roberto A, Castagna L, Zanon V, Bramanti S, Crocchiolo R, McLaren JE, et al. Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood. 2015;125:2855–64.
Martin M, Fornecker LM, Marcellin L, Mousseaux E, Hij A, Snowden JA, et al. Acute and fatal cardiotoxicity following high-dose cyclophosphamide in a patient undergoing autologous stem cell transplantation for systemic sclerosis despite satisfactory cardiopulmonary screening. Bone Marrow Transplant. 2017;52:1674–7.
Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18:1859–66.
Pedraza A, Jorge S, Suárez-Lledó M, Pereira A, Gutiérrez-García G, Fernández-Avilés F, et al. High-dose cyclophosphamide and tacrolimus as graft-versus-host disease prophylaxis for matched and mismatched unrelated donor transplantation. Transplant Cell Ther. 2021;27(619):e1-8.
Carnevale-Schianca F, Caravelli D, Gallo S, Becco P, Paruzzo L, Poletto S, et al. Post-transplant cyclophosphamide and tacrolimus-mycophenolate mofetil combination governs GVHD and immunosuppression need, reducing late toxicities in allogeneic peripheral blood hematopoietic cell transplantation from HLA-matched donors. J Clin Med. 2021;10:1173.
Hagen PA, Adams W, Smith S, Tsai S, Stiff P. Low mean post-transplantation tacrolimus levels in weeks 2–3 correlate with acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation from related and unrelated donors. Bone Marrow Transplant. 2019;54:155–8.
Goto T, Tanaka T, Sawa M, Ueda Y, Ago H, Chiba S, et al. Prospective observational study on the first 51 cases of peripheral blood stem cell transplantation from unrelated donors in Japan. Int J Hematol. 2018;107:211–21.
Acknowledgements
We thank all physicians and data managers who contributed to this study. This work was supported by a grant from the Japan Society for the Promotion of Science (JSPS; KAKENHI Grant number: 17K09017). Author Takahiko Nakane performed this study at Osaka City University Hospital and transferred to the Department of Hematology, Osaka Saiseikai Nakatsu Hospital before publishing this paper.
Funding
This work was supported by a grant from the Japan Society for the Promotion of Science (JSPS; KAKENHI Grant number: 17K09017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
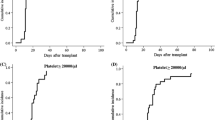
Supplemental Figure. Cumulative incidences of acute and chronic graft-versus-host disease (GVHD) stratified by stem cell source
. Cumulative incidences of (A) grades II–IV and (B) grades III–IV acute GVHD. Cumulative incidences of (C) all and (D) moderate to severe chronic GVHD. rBM and rPBSCs denote HLA-matched related bone marrow and peripheral blood stem cell donors, respectively. uBM and uPBSCs indicate HLA-matched unrelated bone marrow and peripheral blood stem cells donors, respectively. (PPTX 51 KB)
About this article
Cite this article
Nakamae, H., Nakane, T., Okamura, H. et al. A phase II study of post-transplant cyclophosphamide combined with tacrolimus for GVHD prophylaxis after HLA-matched related/unrelated allogeneic hematopoietic stem cell transplantation. Int J Hematol 115, 77–86 (2022). https://doi.org/10.1007/s12185-021-03228-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03228-1