Abstract
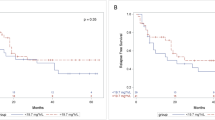
The efficacy of pharmacokinetically (PK) guided, once-daily administration of busulfan (BU) was evaluated in elderly patients with acute myeloid leukemia/myelodysplastic syndrome (AML/MDS). Twenty-one patients (median age 61) received 30 mg/m2 fludarabine for 6 days and BU for 4 days, starting from 3.2 mg/m2 and subsequently adjusted to the target area under the curve (AUC) of 6000 µmol-min/L. The median AUC of day 1 (AUC1), AUC4, and their average were 4871.3, 6021.0, and 5368.1 µmol-min/L, respectively. Veno-occlusive disease/sinusoidal obstructive syndrome (VOD/SOS) occurred in five patients (24%) but all recovered well. Four patients (20%) had non-infectious pulmonary complications (NIPCs). Patients with high AUC1 had frequent gastrointestinal adverse events, but similar incidence of VOD/SOS and NIPCs. Two-year overall survival (OS), non-relapse mortality (NRM), and relapse rates were 44.4%, 28.6%, and 29.1%, respectively. Patients with high AUC1 had significantly high NRM (57.1% vs. 14.3%, P = 0.04) and inferior OS (14.3% vs. 60.1%, P = 0.002), while patients with high AUC4 had a significantly low relapse rate (8.3% vs. 55.6%, P = 0.02). In conclusion, once-daily BU and a PK-guided dose intensification were beneficial for reducing relapse in elderly patients with AML/MDS. However, caution should be exercised as rapid BU dose elevation may contribute to NRM.


Similar content being viewed by others
References
Russell JA, Kangarloo SB, Williamson T, Chaudhry MA, Savoie ML, Turner AR, et al. Establishing a target exposure for once-daily intravenous busulfan given with fludarabine and thymoglobulin before allogeneic transplantation. Biol Blood Marrow Transplant. 2013;19:1381–6.
Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8:477–85.
Geddes M, Kangarloo SB, Naveed F, Quinlan D, Chaudhry MA, Stewart D, et al. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant. 2008;14:220–8.
Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468–76.
Ryu SG, Lee JH, Choi SJ, Lee JH, Lee YS, Seol M, et al. Randomized comparison of four-times-daily versus once-daily intravenous busulfan in conditioning therapy for hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13:1095–105.
Sato M, Kako S, Matsumoto K, Oshima K, Akahoshi Y, Nakano H, et al. Pharmacokinetics study of once-daily intravenous busulfan in conditioning regimens for hematopoietic stem cell transplantation. Int J Hematol. 2015;101:497–504.
Pidala J, Kim J, Anasetti C, Kharfan-Dabaja MA, Field T, Perkins J, et al. Targeted i.v. BU and fludarabine (t-i.v. BU/Flu) provides effective control of AML in adults with reduced toxicity. Bone Marrow Transplant. 2011;46:641–9.
Andersson BS, Thall PF, Valdez BC, Milton DR, Al-Atrash G, Chen J, et al. Fludarabine with pharmacokinetically guided IV busulfan is superior to fixed-dose delivery in pretransplant conditioning of AML/MDS patients. Bone Marrow Transplant. 2017;52:580–7.
Peris JE, Latorre JA, Castel V, Verdeguer A, Esteve S, Torres-Molina F. Determination of busulfan in human plasma using high-performance liquid chromatography with pre-column derivatization and fluorescence detection. J Chromatogr B Biomed Sci Appl. 1999;730:33–40.
Nakamura H, Sato T, Okada K, Miura G, Ariyoshi N, Nakazawa K, et al. Population pharmacokinetics of oral busulfan in young Japanese children before hematopoietic stem cell transplantation. Ther Drug Monit. 2008;30:75–83.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–67.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89:3055–60.
Yeh RF, Pawlikowski MA, Blough DK, McDonald GB, O’Donnell PV, Rezvani A, et al. Accurate targeting of daily intravenous busulfan with 8-hour blood sampling in hospitalized adult hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2012;18:265–72.
Perkins JB, Kim J, Anasetti C, Fernandez HF, Perez LE, Ayala E, et al. Maximally tolerated busulfan systemic exposure in combination with fludarabine as conditioning before allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1099–107.
de Castro FA, Lanchote VL, Voltarelli JC, Colturato VA, Simoes BP. Influence of fludarabine on the pharmacokinetics of oral busulfan during pretransplant conditioning for hematopoietic stem cell transplantation. J Clin Pharmacol. 2013;53:1205–11.
Kikuchi T, Mori T, Ohwada C, Onoda M, Shimizu H, Yokoyama H, et al. Pharmacokinetics of intravenous busulfan as condition for hematopoietic stem cell transplantation: comparison between combinations with cyclophosphamide and fludarabine. Int J Hematol. 2021;113:128–33.
Vassal G. Pharmacologically-guided dose adjustment of busulfan in high-dose chemotherapy regimens: rationale and pitfalls (review). Anticancer Res. 1994;14:2363–70.
McCune JS, Bemer MJ, Barrett JS, Scott Baker K, Gamis AS, Holford NH. Busulfan in infant to adult hematopoietic cell transplant recipients: a population pharmacokinetic model for initial and Bayesian dose personalization. Clin Cancer Res. 2014;20:754–63.
Nguyen L, Leger F, Lennon S, Puozzo C. Intravenous busulfan in adults prior to haematopoietic stem cell transplantation: a population pharmacokinetic study. Cancer Chemother Pharmacol. 2006;57:191–8.
Beumer JH, Owzar K, Lewis LD, Jiang C, Holleran JL, Christner SM, et al. Effect of age on the pharmacokinetics of busulfan in patients undergoing hematopoietic cell transplantation; an alliance study (CALGB 10503, 19808, and 100103). Cancer Chemother Pharmacol. 2014;74:927–38.
Wallen H, Gooley TA, Deeg HJ, Pagel JM, Press OW, Appelbaum FR, et al. Ablative allogeneic hematopoietic cell transplantation in adults 60 years of age and older. J Clin Oncol. 2005;23:3439–46.
O’Donnell PH, Artz AS, Undevia SD, Pai RK, Del Cerro P, Horowitz S, et al. Phase I study of dose-escalated busulfan with fludarabine and alemtuzumab as conditioning for allogeneic hematopoietic stem cell transplant: reduced clearance at high doses and occurrence of late sinusoidal obstruction syndrome/veno-occlusive disease. Leuk Lymphoma. 2010;51:2240–9.
Yakushijin K, Atsuta Y, Doki N, Yokota A, Kanamori H, Miyamoto T, et al. Sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors and outcomes. Bone Marrow Transplant. 2016;51:403–9.
Sakaguchi H, Takahashi Y, Watanabe N, Doisaki S, Muramatsu H, Hama A, et al. Incidence, clinical features, and risk factors of idiopathic pneumonia syndrome following hematopoietic stem cell transplantation in children. Pediatr Blood Cancer. 2012;58:780–4.
Popat U, Mehta RS, Bassett R, Kongtim P, Chen J, Alousi AM, et al. Optimizing the Conditioning Regimen for Hematopoietic Cell Transplant in Myelofibrosis: Long-Term Results of a Prospective Phase II Clinical Trial. Biol Blood Marrow Transplant. 2020;26:1439–45.
Ben-Barouch S, Cohen O, Vidal L, Avivi I, Ram R. Busulfan fludarabine vs busulfan cyclophosphamide as a preparative regimen before allogeneic hematopoietic cell transplantation: systematic review and meta-analysis. Bone Marrow Transplant. 2016;51:232–40.
Weil E, Zook F, Oxencis C, Canadeo A, Urmanski A, Waggoner M, et al. Evaluation of the Pharmacokinetics and Efficacy of a Busulfan Test Dose in Adult Patients Undergoing Myeloablative Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2017;23:952–7.
Beri R, Chunduri S, Sweiss K, Peace DJ, Mactal-Haaf C, Dobogai LC, et al. Reliability of a pretransplant i.v. BU test dose performed 2 weeks before myeloablative FluBu conditioning regimen. Bone Marrow Transplant. 2010;45:249–53.
Acknowledgements
The authors thank our patients and their families who have shared their experiences with us, and Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ohwada, C., Yamazaki, S., Shono, K. et al. Pharmacokinetically guided, once-daily intravenous busulfan in combination with fludarabine for elderly AML/MDS patients as a conditioning regimen for allogeneic stem cell transplantation. Int J Hematol 114, 664–673 (2021). https://doi.org/10.1007/s12185-021-03188-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03188-6