Abstract
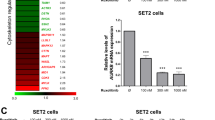
Ruxolitinib (RUX), a JAK1/2-inhibitor, is effective for myeloproliferative neoplasm (MPN) with both JAK2V617 F and calreticulin (CALR) mutations. However, many MPN patients develop resistance to RUX. Although mechanisms of RUX-resistance in cells with JAK2V617 F have already been characterized, those in cells with CALR mutations remain to be elucidated. In this study, we established RUX-resistant human cell lines with CALR mutations and characterized mechanisms of RUX-resistance. Here, we found that RUX-resistant cells had high levels of MPL transcripts, overexpression of both MPL and JAK2, and increased phosphorylation of JAK2 and STAT5. We also found that mature MPL proteins were more stable in RUX-resistant cells. Knockdown of MPL in RUX-resistant cells by shRNAs decreased JAK/STAT signaling. Immunoprecipitation assays showed that binding of mutant CALR to MPL was increased in RUX-resistant cells. Reduction of mutated CALR decreased proliferation of the resistant cells. When resistant cells were cultured in the absence of RUX, the RUX-resistance was reversed, with reduction of the mutant-CALR/MPL complex. In conclusion, MPL overexpression induces higher levels of a mutant-CALR/MPL complex, which may cause RUX-resistance in cells with CALR mutations. This mechanism may be a new therapeutic target to overcome RUX-resistance.







Similar content being viewed by others
References
Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–405. https://doi.org/10.1056/NEJMoa1312542.
Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–90. https://doi.org/10.1056/NEJMoa1311347.
Araki M, Komatsu N. Novel molecular mechanism of cellular transformation by a mutant molecular chaperone in myeloproliferative neoplasms. Cancer Sci. 2017;108(10):1907–12. https://doi.org/10.1111/cas.13327.
Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129(6):667–79. https://doi.org/10.1182/blood-2016-10-695940.
Chachoua I, Pecquet C, El-Khoury M, Nivarthi H, Albu RI, Marty C, et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood. 2016;127(10):1325–35. https://doi.org/10.1182/blood-2015-11-681932.
Elf S, Abdelfattah NS, Chen E, Perales-Paton J, Rosen EA, Ko A, et al. Mutant calreticulin requires both its mutant C-terminus and the thrombopoietin receptor for oncogenic transformation. Cancer Discov. 2016;6(4):368–81. https://doi.org/10.1158/2159-8290.cd-15-1434.
Araki M, Yang Y, Imai M, Mizukami Y, Kihara Y, Sunami Y, et al. Homomultimerization of mutant calreticulin is a prerequisite for MPL binding and activation. Leukemia. 2019;33(1):122–31. https://doi.org/10.1038/s41375-018-0181-2.
Araki M, Yang Y, Masubuchi N, Hironaka Y, Takei H, Morishita S, et al. Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood. 2016;127(10):1307–16. https://doi.org/10.1182/blood-2015-09-671172.
Shide K, Kameda T, Yamaji T, Sekine M, Inada N, Kamiunten A, et al. Calreticulin mutant mice develop essential thrombocythemia that is ameliorated by the JAK inhibitor ruxolitinib. Leukemia. 2017;31(5):1136–44. https://doi.org/10.1038/leu.2016.308.
Marty C, Pecquet C, Nivarthi H, El-Khoury M, Chachoua I, Tulliez M, et al. Calreticulin mutants in mice induce an MPL-dependent thrombocytosis with frequent progression to myelofibrosis. Blood. 2016;127(10):1317–24. https://doi.org/10.1182/blood-2015-11-679571.
Pecquet C, Chachoua I, Roy A, Balligand T, Vertenoeil G, Leroy E, et al. Calreticulin mutants as oncogenic rogue chaperones for TpoR and traffic-defective pathogenic TpoR mutants. Blood. 2019;133(25):2669–81. https://doi.org/10.1182/blood-2018-09-874578.
Masubuchi N, Araki M, Yang Y, Hayashi E, Imai M, Edahiro Y, et al. Mutant calreticulin interacts with MPL in the secretion pathway for activation on the cell surface. Leukemia. 2020;34(2):499–509. https://doi.org/10.1038/s41375-019-0564-z.
Yang LP, Keating GM. Ruxolitinib: in the treatment of myelofibrosis. Drugs. 2012;72(16):2117–27. https://doi.org/10.2165/11209340-000000000-00000.
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–98. https://doi.org/10.1056/NEJMoa1110556.
Guglielmelli P, Rotunno G, Bogani C, Mannarelli C, Giunti L, Provenzano A, et al. Ruxolitinib is an effective treatment for CALR-positive patients with myelofibrosis. Br J Haematol. 2016;173(6):938–40. https://doi.org/10.1111/bjh.13644.
Patel KP, Newberry KJ, Luthra R, Jabbour E, Pierce S, Cortes J, et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood. 2015;126(6):790–7. https://doi.org/10.1182/blood-2015-03-633404.
Harrison CN, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Gisslinger H, Knoops L, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2017;31(3):775. https://doi.org/10.1038/leu.2016.323.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica. 2015;100(4):479–88. https://doi.org/10.3324/haematol.2014.115840.
Cervantes F, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Sirulnik A, Stalbovskaya V, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122(25):4047–53. https://doi.org/10.1182/blood-2013-02-485888.
Verstovsek S, Mesa RA, Gotlib J, Gupta V, DiPersio JF, Catalano JV, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10(1):55. https://doi.org/10.1186/s13045-017-0417-z.
Pardanani A, Tefferi A. How I treat myelofibrosis after failure of JAK inhibitors. Blood. 2018;132(5):492–500. https://doi.org/10.1182/blood-2018-02-785923.
Pardanani A, Tefferi A. Definition and management of ruxolitinib treatment failure in myelofibrosis. Blood Cancer J. 2014;4: e268. https://doi.org/10.1038/bcj.2014.84.
Newberry KJ, Patel K, Masarova L, Luthra R, Manshouri T, Jabbour E, et al. Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood. 2017;130(9):1125–31. https://doi.org/10.1182/blood-2017-05-783225.
Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489(7414):155–9. https://doi.org/10.1038/nature11303.
Deshpande A, Reddy MM, Schade GO, Ray A, Chowdary TK, Griffin JD, et al. Kinase domain mutations confer resistance to novel inhibitors targeting JAK2V617F in myeloproliferative neoplasms. Leukemia. 2012;26(4):708–15. https://doi.org/10.1038/leu.2011.255.
Bhagwat N, Levine RL, Koppikar P. Sensitivity and resistance of JAK2 inhibitors to myeloproliferative neoplasms. Int J Hematol. 2013;97(6):695–702. https://doi.org/10.1007/s12185-013-1353-5.
Springuel L, Hornakova T, Losdyck E, Lambert F, Leroy E, Constantinescu SN, et al. Cooperating JAK1 and JAK3 mutants increase resistance to JAK inhibitors. Blood. 2014;124(26):3924–31. https://doi.org/10.1182/blood-2014-05-576652.
Schuklenk U. Helsinki declaration revisions. Issues Med Ethics. 2001;9(1):29.
Matsumoto N, Mori S, Hasegawa H, Sasaki D, Mori H, Tsuruda K, et al. Simultaneous screening for JAK2 and calreticulin gene mutations in myeloproliferative neoplasms with high resolution melting. Clin Chim Acta. 2016;462:166–73. https://doi.org/10.1016/j.cca.2016.09.023.
Shirane S, Araki M, Morishita S, Edahiro Y, Takei H, Yoo Y, et al. JAK2, CALR, and MPL mutation spectrum in Japanese patients with myeloproliferative neoplasms. Haematologica. 2015;100(2):e46–8. https://doi.org/10.3324/haematol.2014.115113.
Komatsu N, Yamamoto M, Fujita H, Miwa A, Hatake K, Endo T, et al. Establishment and characterization of an erythropoietin-dependent subline, UT-7/Epo, derived from human leukemia cell line, UT-7. Blood. 1993;82(2):456–64.
Kurien BT, Scofield RH. Western blotting. Methods. 2006;38(4):283–93. https://doi.org/10.1016/j.ymeth.2005.11.007.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–8. https://doi.org/10.1006/meth.2001.1262.
Black L, Berenbaum MC. Factors affecting the dye exclusion test for cell viability. Exp Cell Res. 1964;35:9–13.
Hamilton VT, Habbersett MC, Herman CJ. Flow microfluorometric analysis of cellular DNA: critical comparison of mithramycin and propidium iodide. J Histochem Cytochem. 1980;28(10):1125–8. https://doi.org/10.1177/28.10.6448270.
Saur SJ, Sangkhae V, Geddis AE, Kaushansky K, Hitchcock IS. Ubiquitination and degradation of the thrombopoietin receptor c-Mpl. Blood. 2010;115(6):1254–63. https://doi.org/10.1182/blood-2009-06-227033.
Akiyama H, Umezawa Y, Watanabe D, Okada K, Ishida S, Nogami A, et al. Inhibition of USP9X downregulates JAK2-V617F and induces apoptosis synergistically with BH3 mimetics preferentially in ruxolitinib-persistent JAK2-V617F-positive leukemic cells. Cancers. 2020. https://doi.org/10.3390/cancers12020406.
Drachman JG, Griffin JD, Kaushansky K. The c-Mpl ligand (thrombopoietin) stimulates tyrosine phosphorylation of Jak2, Shc, and c-Mpl. J Biol Chem. 1995;270(10):4979–82. https://doi.org/10.1074/jbc.270.10.4979.
Moliterno AR, Hankins WD, Spivak JL. Impaired expression of the thrombopoietin receptor by platelets from patients with polycythemia vera. N Engl J Med. 1998;338(9):572–80. https://doi.org/10.1056/nejm199802263380903.
Moliterno AR, Spivak JL. Posttranslational processing of the thrombopoietin receptor is impaired in polycythemia vera. Blood. 1999;94(8):2555–61.
Besancenot R, Roos-Weil D, Tonetti C, Abdelouahab H, Lacout C, Pasquier F, et al. JAK2 and MPL protein levels determine TPO-induced megakaryocyte proliferation vs differentiation. Blood. 2014;124(13):2104–15. https://doi.org/10.1182/blood-2014-03-559815.
Pradhan A, Lambert QT, Griner LN, Reuther GW. Activation of JAK2-V617F by components of heterodimeric cytokine receptors. J Biol Chem. 2010;285(22):16651–63. https://doi.org/10.1074/jbc.M109.071191.
Lu X, Huang LJ, Lodish HF. Dimerization by a cytokine receptor is necessary for constitutive activation of JAK2V617F. J Biol Chem. 2008;283(9):5258–66. https://doi.org/10.1074/jbc.M707125200.
Bandaranayake RM, Ungureanu D, Shan Y, Shaw DE, Silvennoinen O, Hubbard SR. Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F. Nat Struct Mol Biol. 2012;19(8):754–9. https://doi.org/10.1038/nsmb.2348.
Wilmes S, Hafer M, Vuorio J, Tucker JA, Winkelmann H, Löchte S, et al. Mechanism of homodimeric cytokine receptor activation and dysregulation by oncogenic mutations. Science. 2020;367(6478):643–52. https://doi.org/10.1126/science.aaw3242.
Han L, Schubert C, Köhler J, Schemionek M, Isfort S, Brümmendorf TH, et al. Calreticulin-mutant proteins induce megakaryocytic signaling to transform hematopoietic cells and undergo accelerated degradation and Golgi-mediated secretion. J Hematol Oncol. 2016;9(1):45. https://doi.org/10.1186/s13045-016-0275-0.
Acknowledgements
We thank Ms. Okada and Ms. Liu Meiou for technical supports. We thank Dr. Nami Masubuchi for her supports for qPCR experiments. We thank Dr. Toshiaki Ohteki and Dr. Masashi Kanayama in Department of Biodefence Research, Medical Research Institute at TMDU for their supports for animal experiments. We also thank Ms. Inoue’s help for IHC staining. This research was supported by Nagao-Takeshi Research grant (Yoshihiro Umezawa) and TMDU Young Investigator Research grant (Yoshihiro Umezawa).
Author information
Authors and Affiliations
Contributions
SY, SA, DW, MA, OM and NKa designed the experiments. SY, RY, HL, HA, MI and MA performed the experiments. SY, YE, ES, NKo, YN, ND and NKa contributed to collection of clinical samples. SY, RY, HL and KY examined histopathological samples. SY, SA, MA, ES, YN, ND and NKa analyzed the data. SY, TF and NKa performed statistical analysis. SY, SA, MA, NKo, OM and NKa wrote the paper. All the authors have approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All of the authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yasuda, S., Aoyama, S., Yoshimoto, R. et al. MPL overexpression induces a high level of mutant-CALR/MPL complex: a novel mechanism of ruxolitinib resistance in myeloproliferative neoplasms with CALR mutations. Int J Hematol 114, 424–440 (2021). https://doi.org/10.1007/s12185-021-03180-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03180-0