Abstract
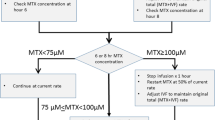
High-dose methotrexate (HD-MTX) therapy is widely used in patients with acute lymphoblastic leukemia (ALL) and lymphoma. However, some patients experience delayed MTX elimination, which requires treatment suspension or dose reduction to avoid organ damage. This single-center retrospective analysis reviewed the clinical data of 88 children with ALL or non-Hodgkin lymphoma who received a total of 269 courses of HD-MTX therapy between April 2008 and April 2019. HD-MTX was defined as MTX administration at 2.0, 3.0, or 5.0 g/m2 over a 24-h period, and delayed MTX elimination was defined as a serum MTX concentration ≥ 1.0 µmol/L at 48 h after the start of HD-MTX. Clinical factors were compared between courses with and without delayed MTX elimination. MTX elimination was delayed in 21 of the 269 courses (7.8%). Multivariate analysis showed that first HD-MTX course (OR 4.04), lower urine volume per BSA on the first day of HD-MTX administration (< 2,675 mL/m2, OR 5.10), higher total bilirubin (> 0.5 mg/dL, OR 5.11), lower eGFR (< 136 mL/min/1.73 m2, OR 3.90), higher dose of MTX(> 3.0 g/m2, OR 10.8), and lower urine volume per BSA on the next day of starting HD-MTX (< 2,107 mL/m2, OR 3.43) were independent risk factors for delayed MTX elimination.

Similar content being viewed by others
References
Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471–82.
Petez C, Wang YM, Sutow WW, Herson J. Significance of the 48-hour plasma level in high-dose methotrexate regimens. Cancer Clin Trials. 1978;1(2):107–11.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Uemura O, Nagai T, Ishikura K, Ito S, Hataya H, Gotoh Y, Fujita N, Akioka Y, Kaneko T, Honda M. Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol. 2014;18:626–33.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Yanagimachi M, Goto H, Kaneko T, Naruto T, Sasaki K, Takeuchi M, Tanoshima R, Kato H, Yokosuka T, Kajiwara R, Fujii H, Tanaka F, Goto S, Takahashi H, Mori M, Kai S, Yokota S. Influence of pre-hydration and pharmacogenetics on plasma methotrexate concentration and renal dysfunction following high-dose methotrexate therapy. Int J Hematol. 2013;98:702–7.
Suzuki K, Doki K, Homma M, Tamaki H, Hori S, Ohtani H, Sawada Y, Kohda Y. Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol. 2009;67:44–9.
Yamamoto K, Sawada Y, Matsushita Y, Moriwaki K, Bessho F, Iga T. Delayed elimination of methotrexate associated with piperacillin administration. Ann Pharmacother. 1997;31:1261–2.
de Miguel D, García-Suárez J, Martín Y, Gil-Fernández JJ, Burgaleta C. Severe acute renal failure following high-dose methotrexate therapy in adults with hematological malignancies: a significant number result from unrecognized co-administration of several drugs. Nephrol Dial Transplant. 2008;23:3762–6.
Pauley JL, Panetta JC, Schmidt J, Kornegay N, Relling MV, Pui CH. Late-onset delayed excretion of methotrexate. Cancer Chemother Pharmacol. 2004;54:146–52.
Wright KD, Panetta JC, Onar-Thomas A, Reddick WE, Patay Z, Qaddoumi I, Broniscer A, Robinson G, Boop FA, Klimo P Jr, Ward D, Gajjar A, Stewart CF. Delayed methotrexate excretion in infants and young children with primary central nervous system tumors and postoperative fluid collections. Cancer Chemother Pharmacol. 2015;75:27–35.
Samantha R, Larry B, Nelly A, Debra A, Sean M, Dan D. Hypoalbminemia is sgnificantly associated with increased clearance time of high dose methotrexate in patients being treated for lymphoma or leukemia. Ann Hematol. 2016;95:2009–15.
Hall JJ, Bolina M, Chatterley T, Jamali F. Interaction between low-dose methotrexate and nonsteroidal anti-inflammatory drugs, penicillins, and proton pump inhibitors. Ann Pharmacother. 2017;51:163–78.
Sasaki K, Tanaka J, Fujimoto T. Theoretically required urinary flow during high-dose methotrexate infusion. Cancer Chemother Pharmacol. 1984;13:9–13.
Xu WQ, Zhang LY, Chen XY, Pan BH, Mao JQ, Song H, Li JY, Tang YM. Serum creatinine and creatinine clearance for predicting plasma methotrexate concentrations after high-dose methotrexate chemotherapy for the treatment for childhood lymphoblastic malignancies. Cancer Chemother Pharmacol. 2014;73:79–86.
Skärby T, Jönsson P, Hjorth L, Behrentz M, Björk O, Forestier E, Jarfelt M, Lönnerholm G, Höglund P. High-dose methotrexate: on the relationship of methotrexate elimination time vs renal function and serum methotrexate levels in 1164 courses in 264 Swedish children with acute lymphoblastic leukaemia (ALL). Cancer Chemother Pharmacol. 2003;51:311–20.
Joannon P, Oviedo I, Campbell M, Tordecilla J. High-dose methotrexate therapy of childhood acute lymphoblastic leukemia: lack of relation between serum methotrexate concentration and creatinine clearance. Pediatr Blood Cancer. 2004;43:17–22.
Xu W, Tang Y, Song H, Shi S, Yang S. Retrospective study on elimination delay of methotrexate in high-dose therapy of childhood acute lymphoblastic leukemia in China. J Pediatr Hematol Oncol. 2007;29:688–93.
Laila H, Anders M, Lars M, Lars S, Kirsten S. High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. Br J Clin Pharmacol. 2012;73:106–14.
Sterba J, Dusek L, Demlova R, Valik D. Pretreatment plasma folate modulates the pharmacodynamics effect of high-dose methotrexate in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma: ‘folate over rescue’ concept revisited. Clin Chem. 2006;52:692–700.
Valik D, Sterba J, Bajciova V, Demlova R. Severe encephalopathy induced by the first but not the second course of high-dose methotrexate mirrored by plasma homocysteine elevations and preceded by extreme differences in pretreatment plasma folate. Oncology. 2005;69:269–72.
Tsurusawa M, Gosho M, Mori T, Mitsui T, Sunami S, Kobayashi R, Fukano R, Tanaka F, Fujita N, Inada H, Koh K, Takimoto T, Saito A, Fujimoto J, Nakazawa A, Horibe K; lymphoma committee of the Japanese Pediatric Leukemia/lymphoma Study Group. Statistical analysis of relation between plasma methotrexate concentration and toxicity in high-dose methotrexate therapy of childhood non-Hodgkin lymphoma. Pediatr Blood Cancer. 2015; 62:279–284.
Locasciulli A, Mura R, Fraschini D, Gornati G, Scovena E, Gervasoni A, Uderzo C, Masera G. High-dose methotrexate administration and acute liver damage in children treated for acute lymphoblastic leukemia. A prospective study. Haematologica. 1992;77(1):49–53.
Innocenti F, Danesi R, Di Paolo A, Loru B, Favre C, Nardi M, Bocci G, Nardini D, Macchia P, Del Tacca M. Clinical and experimental pharmacokinetic interaction between 6-mercaptopurine and methotrexate. Cancer Chemother Pharmacol. 1996;37:409–14.
Toshiaki T, Atsushi Y, Kiyoshi I. Reference intervals of clinical tests in children determined by a latent reference value extraction method. J Jpn Pediatr Soc. 2008;112:1117–32.
Drost SA, Wentzell JR, Giguère P, McLurg DL, Sabloff M, Kanji S, Nguyen TT. Outcomes associated with reducing the urine alkalinization threshold in patients receiving high-dose methotrexate. Pharmacotherapy. 2017;37:684–91.
Ramsey LB, Panetta JC, Smith C, et al. Genomewide study of methotrexate clearance replicates SLCO1B1. Blood. 2013;121:898–904.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nakano, T., Kobayashi, R., Matsushima, S. et al. Risk factors for delayed elimination of high-dose methotrexate in childhood acute lymphoblastic leukemia and lymphoma. Int J Hematol 113, 744–750 (2021). https://doi.org/10.1007/s12185-020-03071-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-03071-w