Abstract
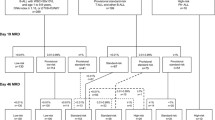
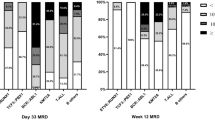
Acute lymphoblastic leukemia (ALL) is a heterogeneous disease whose prognostic factors include minimal residual disease (MRD) and cytogenetic abnormalities. To explore the significance of MRD in ALL subtypes, we analyzed the outcomes of 1126 children treated with risk-stratified therapy based on sequential MRD monitoring. MRD distributions and treatment outcomes differed between distinct leukemia subtypes. Patients with ETV6-RUNX1 or hyperdiploidy had the best prognosis (5-year OS: 97 ± 1.5% and 89.2 ± 2.7%). However, hyperdiploidy patients with MRD ≥ 10% on day 15 had a higher risk of relapse (36.4%) than those with ETV6-RUNX1. TCF3-PBX1 patients had the fastest disease clearance (negative MRD rate on day 33: 92.1%), but the overall prognosis was intermediate (5-year OS: 82.5%). Patients with high-risk characteristics and ALL-T had inferior outcomes: even with undetectable MRD on day 33, cumulative incidence of relapse was 19.9% and 23.4%, respectively. Moreover, those with poor early-treatment response and detectable week-12 MRD had a worse prognosis. After adjusting for other risk factors, re-emergent MRD was the most significant adverse prognostic indicator overall. Sequential MRD measurement is important for MRD-guided therapy, and integration of MRD values at different timepoints based on leukemia subtype could allow for more refined risk stratification.



Similar content being viewed by others
References
Bonaventure A, Harewood R, Stiller CA, Gatta G, Clavel J, Stefan DC, et al. Worldwide comparison of survival from childhood leukaemia for 1995–2009, by subtype, age, and sex (CONCORD-2): a population-based study of individual data for 89 828 children from 198 registries in 53 countries. Lancet Haematol. 2017;4:e202–17.
Campana D, Pui CH. Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood. 2017;129:1913–8.
Berry DA, Zhou S, Higley H, Mukundan L, Fu S, Reaman GH, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. Jama Oncol. 2017;3:e170580.
Pieters R, de Groot-Kruseman H, Van der Velden V, Fiocco M, van den Berg H, de Bont E, et al. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: study all10 from the dutch childhood oncology group. J Clin Oncol. 2016;34:2591–601.
Pui CH, Pei D, Coustan-Smith E, Jeha S, Cheng C, Bowman WP, et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol. 2015;16:465–74.
Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33:2938–48.
Pui CH, Pei D, Campana D, Cheng C, Sandlund JT, Bowman WP, et al. A revised definition for cure of childhood acute lymphoblastic leukemia. Leukemia. 2014;28:2336–43.
Vora A, Goulden N, Mitchell C, Hancock J, Hough R, Rowntree C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15:809–18.
Pui CH, Pei D, Raimondi SC, Coustan-Smith E, Jeha S, Cheng C, et al. Clinical impact of minimal residual disease in children with different subtypes of acute lymphoblastic leukemia treated with response-adapted therapy. Leukemia. 2017;31:333–9.
Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia. 1995;9:1783–6.
Pui CH, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 1998;339:605–15.
Qin Y, Zhu H, Jiang B, Li J, Lu X, Li L, et al. Expression patterns of WT1 and PRAME in acute myeloid leukemia patients and their usefulness for monitoring minimal residual disease. Leuk Res. 2009;33:384–90.
Qin YZ, Zhu HH, Liu YR, Wang YZ, Shi HX, Lai YY, et al. PRAME and WT1 transcripts constitute a good molecular marker combination for monitoring minimal residual disease in myelodysplastic syndromes. Leuk Lymphoma. 2013;54:1442–9.
Cui L, Li ZG, Chai YH, et al. Outcome of children with newly diagnosed acute lymphoblastic leukemia treated with CCLG-ALL 2008: The first nation-wide prospective multicenter study in China. Am J Hematol. 2018;93:913–20.
Liu YR, Zhang LP, Chang Y, Cheng YF, Fu JY, Li LD, et al. (2006) Clinical significance for minimal residual disease detection by 4 color flow cytometry in adult and childhood B lineage acute lymphoblastic leukemia. Zhonghua Xue Ye Xue Za Zhi 27:302–305.
Zhao XS, Liu YR, Zhu HH, Xu LP, Liu DH, Liu KY, et al. Monitoring MRD with flow cytometry: an effective method to predict relapse for ALL patients after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2012;91:183–92.
Xue YJ, Suo P, Huang XJ, Lu AD, Wang Y, Zuo YX, et al. Superior survival of unmanipulated haploidentical haematopoietic stem cell transplantation compared with intensive chemotherapy as post-remission treatment for children with very high-risk philadelphia chromosome negative B-cell acute lymphoblastic leukaemia in first complete remission. Br J Haematol. 2020;188:757–67.
Winick N, Devidas M, Chen S, Maloney K, Larsen E, Mattano L, et al. Impact of initial CSF findings on outcome among patients with national cancer institute standard- and high-risk b-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2017;35:2527–34.
Yeoh AE, Ariffin H, Chai EL, Kwok CS, Chan YH, Ponnudurai K, et al. Minimal residual disease-guided treatment deintensification for children with acute lymphoblastic leukemia: results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 study. J Clin Oncol. 2012;30:2384–92.
Toft N, Birgens H, Abrahamsson J, Griskevicius L, Hallbook H, Heyman M, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia. 2018;32:606–15.
Takahashi H, Kajiwara R, Kato M, Hasegawa D, Tomizawa D, Noguchi Y, et al. Treatment outcome of children with acute lymphoblastic leukemia: the Tokyo Children’s Cancer Study Group (TCCSG) Study L04–16. Int J Hematol. 2018;108:98–108.
Pemmaraju N, Kantarjian H, Jorgensen JL, Jabbour E, Jain N, Thomas D, et al. Significance of recurrence of minimal residual disease detected by multi-parameter flow cytometry in patients with acute lymphoblastic leukemia in morphological remission. Am J Hematol. 2017;92:279–85.
Del PM, Buzzatti E, Piciocchi A, Forghieri F, Bonifacio M, Lessi F, et al. Clinical significance of occult central nervous system disease in adult acute lymphoblastic leukemia. HAEMATOLOGICA: A multicenter report from the campus all network; 2019.
Taskinen M, Oskarsson T, Levinsen M, Bottai M, Hellebostad M, Jonsson OG, et al. The effect of central nervous system involvement and irradiation in childhood acute lymphoblastic leukemia: Lessons from the NOPHO ALL-92 and ALL-2000 protocols. Pediatr Blood Cancer. 2017;64:242–9.
Schrappe M, Hunger SP, Pui CH, Saha V, Gaynon PS, Baruchel A, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med. 2012;366:1371–81.
O’Connor D, Enshaei A, Bartram J, Hancock J, Harrison CJ, Hough R, et al. Genotype-Specific Minimal Residual Disease Interpretation Improves Stratification in Pediatric Acute Lymphoblastic Leukemia. J Clin Oncol. 2018;36:34–43.
Gao C, Zhao XX, Li WJ, Cui L, Zhao W, Liu SG, et al. Clinical features, early treatment responses, and outcomes of pediatric acute lymphoblastic leukemia in China with or without specific fusion transcripts: a single institutional study of 1,004 patients. Am J Hematol. 2012;87:1022–7.
Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–41.
Biondi A, Schrappe M, De Lorenzo P, Castor A, Lucchini G, Gandemer V, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13:936–45.
Schultz KR, Carroll A, Heerema NA, Bowman WP, Aledo A, Slayton WB, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia. 2014;28:1467–71.
Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grümayer R, Möricke A, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206–14.
Acknowledgements
This work was supported by the Foundation of 2018 Beijing Key Clinical Specialty Construction Project-Pediatrics (2199000726).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Xue, Yj., Wang, Y., Jia, Yp. et al. The role of minimal residual disease in specific subtypes of pediatric acute lymphoblastic leukemia. Int J Hematol 113, 547–555 (2021). https://doi.org/10.1007/s12185-020-03063-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-03063-w