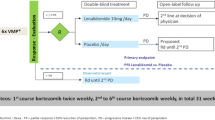
Abstract
We compared efficacy and safety, according to frailty, of elderly patients with relapsed and refractory multiple myeloma (RRMM) treated with lenalidomide and dexamethasone (Rd), for whom bortezomib treatment had failed. Patients, 164 (52.9%) and 146 (47.1%), were classified as non-frail and frail using a simplified frailty scale. The overall response rates (ORR) and survival outcomes were lower in frail than in non-frail patients (ORR: 56.2% vs. 67.7%, P = 0.069; median progression free survival: 13.17 vs. 17.80 months, P = 0.033; median overall survival: 23.00 vs. 36.27 months, P = 0.002, respectively). The number of treatment emergent adverse events in grade 3 or worse was higher in frail than in non-frail patients (41.8% vs. 24.4%, P = 0.002, respectively). In frail patients, independent poor prognostic factors for survival were two or more Charlson comorbidity index (CCI) score, prior to exposure to both bortezomib and thalidomide, and achieved less than partial response In conclusion, frailty could predict clinical outcomes of Rd treatment in elderly patients with RRMM who had failed prior bortezomib. In frail patients, lower CCI in addition to less previous treatment exposure and deep response were associated with better survival.

Similar content being viewed by others
References
Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60.
Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–72.
Warren JL, Harlan LC, Stevens J, Little RF, Abel GA. Multiple myeloma treatment transformed: a population-based study of changes in initial management approaches in the United States. J Clin Oncol. 2013;31:1984–9.
Bringhen S, Mateos MV, Zweegman S, Larocca A, Falcone AP, Oriol A, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013;98:980–7.
Cardoso AL, Fernandes A, Aguilar-Pimentel JA, de Angelis MH, Guedes JR, Brito MA, et al. Towards frailty biomarkers: candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214–77.
Larocca A, Dold SM, Zweegman S, Terpos E, Wäsch R, D’Agostino M, et al. Patient-centered practice in elderly myeloma patients: an overview and consensus from the European Myeloma Network (EMN). Leukemia. 2018;32:1697–712.
Sonneveld P. Management of multiple myeloma in the relapsed/refractory patient. Hematology. 2017;2017:508–17.
Leng S, Bhutani D, Lentzsch S. How I treat a refractory myeloma patient who is not eligible for a clinical trial. Hematology. 2019;9:125–36.
Moreau P, San Miguel J, Sonneveld P, Mateos M-V, Zamagni E, Avet-Loiseau H, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv52–iv61.
Dingli D, Ailawadhi S, Bergsagel PL, Buadi FK, Dispenzieri A, Fonseca R, et al. Therapy for relapsed multiple myeloma: guidelines from the mayo stratification for myeloma and risk-adapted therapy. Mayo Clin Proc. 2017;92:578–98.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–52.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–31.
Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–34.
Engelhardt M, Domm A-S, Dold SM, Ihorst G, Reinhardt H, Zober A, et al. A concise revised myeloma comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017;102:910–21.
Engelhardt M, Dold SM, Ihorst G, Zober A, Möller M, Reinhardt H, et al. Geriatric assessment in multiple myeloma patients: validation of the International Myeloma Working Group (IMWG) score and comparison with other common comorbidity scores. Haematologica. 2016;101:1110–9.
Facon T, Dimopoulos MA, Meuleman N, Belch A, Mohty M, Chen W-M, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia. 2020;34:224–33.
Palumbo A, Bringhen S, Mateos M-V, Larocca A, Facon T, Kumar SK, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–74.
Jo J-C, Lee HS, Kim K, Lee J-J, Yoon S-S, Bang S-M, et al. The effectiveness and safety of lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma in real-world clinical practice: a study of the Korean Multiple Myeloma Working Party (KMMWP-151 study). Ann Hematol. 2020;99:309–19.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e346346.
Gonsalves W, Leung N, Rajkumar SV, Dispenzieri A, Lacy M, Hayman S, et al. Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2015;5:e296.
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9.
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955–62.
Bringhen S, Offidani M, Palmieri S, Pisani F, Rizzi R, Spada S, et al. Early mortality in myeloma patients treated with first-generation novel agents thalidomide, lenalidomide, bortezomib at diagnosis: a pooled analysis. Crit Rev Oncol Hemat. 2018;130:27–35.
Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–42.
Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau J-L, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–32.
Cook G, Larocca A, Facon T, Zweegman S, Engelhardt M. Defining the vulnerable patient with myeloma—a frailty position paper of the European Myeloma Network. Leukemia. 2020;1:10.
Acknowledgements
The authors would like to thank the Korean Multiple Myeloma Working Party and all the researchers and research nurses for their help in data collection and Sangjin Lee who analyzed data statistically. We would like to thank Editage (www.editage.co.kr) for English language editing.
Author information
Authors and Affiliations
Consortia
Contributions
All authors had access to primary clinical trial data. This study was planned and led by Chang-Ki Min, written by Ho Sup Lee, and analyzed statistically by Sangjin Lee. Patient information was provided by the many investigators of the KMMWP. All authors were involved in manuscript preparation, which was led by Ho Sup Lee and Chang-Ki Min, and all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest. No funding was received for this study.
Ethical approval
According to the Declaration of Helsinki, the trial was approved by the Institutional Research Ethics Board of Kosin University Gospel Hospital, which waived the requirement for informed consent given the retrospective nature of this study (IRB approval number 2020–01-005).
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Lee, H.S., Kim, K., Lee, JJ. et al. Clinical impact of frailty on treatment outcomes of elderly patients with relapsed and/or refractory multiple myeloma treated with lenalidomide plus dexamethasone. Int J Hematol 113, 81–91 (2021). https://doi.org/10.1007/s12185-020-02988-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-02988-6