Abstract
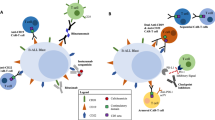
Novel therapies are needed for children with relapsed/refractory (R/R) B-cell precursor acute lymphoblastic leukemia (ALL). Blinatumomab is a bispecific T-cell engager immunotherapy that simultaneously binds to CD3-positive cytotoxic T cells and CD19-positive B cells and redirects the patient’s T cells to lyse malignant and normal B cells. We conducted an open-label phase 1b study to determine the safety, pharmacokinetics, efficacy, and recommended dose of blinatumomab in Japanese children with R/R B-cell precursor ALL. Patients received induction blinatumomab for 4 weeks (5 μg/m2/day week 1; 15 μg/m2/day weeks 2–4), followed by a 2-week treatment-free interval (6-week cycle). In subsequent cycles, patients received blinatumomab 15 μg/m2/day. The primary end point was the incidence of dose-limiting toxicities. Nine patients received blinatumomab. Since no dose-limiting toxicities were reported, the maximum tolerated dose was 5 μg/m2/day for week 1, followed by 15 μg/m2/day weeks 2–4 (5–15 μg/m2/day, the global recommended dose of blinatumomab). All patients had ≥ 1 grade ≥ 3 adverse events; 89% had grade ≥ 3 treatment-related adverse events. M1 remission rate within the first two cycles of treatment was 56%; one patient had a minimal residual disease response. Consistent with global studies, blinatumomab appeared to be safe with preliminary evidence of efficacy in Japanese children with R/R B-cell precursor ALL.




Similar content being viewed by others
Data sharing statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at https://www.amgen.com/datasharing.
References
Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33:2938–48.
Ko RH, Ji L, Barnette P, Bostrom B, Hutchinson R, Raetz E, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. 2010;28:648–54.
Von Stackelberg A, Völzke E, Kühl JS, Seeger K, Schrauder A, Escherich G, et al. Outcome of children and adolescents with relapsed acute lymphoblastic leukaemia and non-response to salvage protocol therapy: a retrospective analysis of the ALL-REZ BFM Study Group. Eur J Cancer. 2011;47:90–7.
Foster JB, Maude SL. New developments in immunotherapy for pediatric leukemia. Curr Opin Pediatr. 2018;30:25–9.
Löffler A, Gruen M, Wuchter C, Schriever F, Kufer P, Dreier T, et al. Efficient elimination of chronic lymphocytic leukaemia B cells by autologous T cells with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Leukemia. 2003;17:900–9.
Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–7.
Piccaluga PP, Arpinati M, Candoni A, Laterza C, Paolini S, Gazzola A, et al. Surface antigens analysis reveals significant expression of candidate targets for immunotherapy in adult acute lymphoid leukemia. Leuk Lymphoma. 2011;52:325–7.
Raponi S, De Propris MS, Intoppa S, Milani ML, Vitale A, Elia L, et al. Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma. 2011;52:1098–107.
Von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34:4381–9.
Nägele V, Kratzer A, Zugmaier G, Holland C, Hijazi Y, Topp MS, et al. Changes in clinical laboratory parameters and pharmacodynamic markers in response to blinatumomab treatment of patients with relapsed/refractory ALL. Exp Hematol Oncol. 2017;6:14.
Zhu M, Wu B, Brandl C, Johnson J, Wolf A, Chow A, et al. Blinatumomab, a bispecific T-cell engager (BiTE®) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. 2016;55:1271–88.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300.
Locatelli F, Zugmaier G, Bader P, Jeha S, Schlegel P-G, Bourquin J-P, et al. High molecular remission rate in pediatric patients (pts) with relapsed/refractory B-cell precursor acute lymphoblastic leukemia (R/R all) treated with blinatumomab: Rialto an open-label, multicenter, expanded access study. Blood. 2018;132:1375.
Kiyoi H, Morris JD, Oh I, Maeda Y, Minami H, Miyamoto T, et al. Phase 1b/2 study of blinatumomab in Japanese adults with relapsed/refractory acute lymphoblastic leukemia. Cancer Sci. 2020;111:1314–23.
Klinger M, Brandl C, Zugmaier G, Hijazi Y, Bargou RC, Topp MS, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119:6226–333.
Zhu M, Kratzer A, Johnson J, Holland C, Brandl C, Singh I, et al. Blinatumomab pharmacodynamics and exposure-response relationships in relapsed/refractory acute lymphoblastic leukemia. J Clin Pharmacol. 2018;58:168–79.
Acknowledgements
Writing support was funded by Amgen Inc. and was provided by Kathryn Boorer, PhD, of KB Scientific Communications, LLC. The authors would like to thank Min Zhu for assistance with the pharmacokinetic analysis.
Author information
Authors and Affiliations
Contributions
Study conception and design: JDM, CO, HG. Data collection and analysis were performed by: KH, CDS, JK, AA, CAT. Manuscript drafts were written and reviewed by all authors. The funder contributed to study design, data collection, data analysis, and data interpretation, and funded a professional medical writer to assist with writing the report. The authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Conflict of interest
This clinical trial was funded by Amgen Inc. and Amgen Astellas BioPharma K. K. (ClinicalTrials.gov: NCT02412306). KH, HG, and CO are advisory board members of Amgen Inc. JDM, CAT, and AA are employees and stockholders of Amgen Inc. CDS and JK are former employees and stockholders of Amgen Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Horibe, K., Morris, J.D., Tuglus, C.A. et al. A phase 1b study of blinatumomab in Japanese children with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Int J Hematol 112, 223–233 (2020). https://doi.org/10.1007/s12185-020-02907-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-02907-9