Abstract
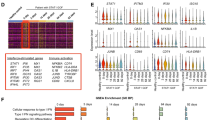
Regulatory T-cells (Tregs) are major mediators of mammalian self-tolerance via cytotoxic T-lymphocyte antigen 4 (CTLA4) signaling pathways. An immune dysregulation syndrome associated with heterozygous germline mutations in CTLA4 was recently reported. Clinical features include recurrent infections, systemic lymphadenopathy, various autoimmune conditions, hypogammaglobulinemia, and autosomal dominant inheritance, characteristic of primary immunodeficient disease (PID). PID symptoms are variable and few patients with sporadic de novo CTLA4 germline mutations have been described. Here, we report the case of a 26-year-old man with an immune dysregulation syndrome and a de novo CTLA4 germline mutation. The patient exhibited several clinical features associated with PID. Next-generation sequencing revealed a CTLA4 germline mutation, c.436G>A; p.G146R, in exon 2 of CTLA4. Sanger sequencing confirmed the patient was the only member of his family with this germline mutation. The patient was diagnosed with an immune dysregulation syndrome associated with de novo germline CTLA4 mutation, complicated by steroid-refractory rheumatoid arthritis. Treatment with abatacept, a CTLA4-immunoglobulin fusion molecule, was initiated, resulting in dramatic resolution of the patient’s clinical symptoms. As PID with CTLA4 germline mutation is rare and patients may be under-diagnosed, physicians should be aware of the features of PID.


Similar content being viewed by others
References
Gutierrez-Arcelus M, Rich SS, Raychaudhuri S. Autoimmune diseases—connecting risk alleles with molecular traits of the immune system. Nat Rev Genet. 2016;17:160–74.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–18.
Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, et al. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med. 2009;206:421–34.
Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32.
Bacchetta R, Notarangelo LD. Immunodeficiency with autoimmunity: beyond the paradox. Front Immunol. 2013;4:3389.
Zeissig S, Petersen B-S, Tomczak M, Melum E, Huc-Claustre E, Dougan SK, et al. Early-onset Crohn’s disease and autoimmunity associated with a variant in CTLA-4. Gut. 2015;64:1889–97.
Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20:1410–6.
Avery DT, Seroogy CM, Tran DQ, Frucht DM, Lenardo MJ, Holland SM, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–7.
Schwab C, Gabrysch A, Olbrich P, Patiño V, Warnatz K, Wolff D, et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4-insufficient subjects. J Allergy Clin Immunol. 2018;142:1932–46.
Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–15.
Genovese MC, Becker J-C, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114–23.
Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Pérez N, Silva CA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383–91.
Lo B, Zhang K, Lu W, Zheng L, Zhang Q, Kanellopoulou C, et al. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349:436–40.
McCusker C, Upton J, Warrington R. Primary immunodeficiency. Allergy Asthma Clin Immunol. 2018;14:61.
O’Keefe AW, Halbrich M, Ben-Shoshan M, McCusker C. Primary immunodeficiency for the primary care provider. Paediatr Child Health. 2016;21:e10–4.
Tsujita Y, Mitsui-Sekinaka K, Imai K, Yeh TW, Mitsuiki N, Asano T, et al. Phosphatase and tensin homolog (PTEN) mutation can cause activated phosphatidylinositol 3-kinase δ syndrome–like immunodeficiency. J Allergy Clin Immunol. 2016;138(1672–1680):e10.
Lien R, Lin YF, Lai MW, Weng HY, Wu RC, Jaing TH, et al. Novel mutations of the tetratricopeptide repeat domain 7A gene and phenotype/genotype comparison. Front Immunol. 2017;8:1–10.
Yamashita M, Wakatsuki R, Kato T, Okano T, Yamanishi S, Mayumi N, et al. A synonymous splice site mutation in IL2RG gene causes late-onset combined immunodeficiency. Int J Hematol. 2019;109:603–11.
Fang M, Abolhassani H, Lim CK, Zhang J, Hammarström L. Next generation sequencing data analysis in primary immunodeficiency disorders—future directions. J Clin Immunol. 2016;36:68–75.
Slatter MA, Engelhardt KR, Burroughs LM, Arkwright PD, Nademi Z, Skoda-Smith S, et al. Hematopoietic stem cell transplantation for CTLA4 deficiency. J Allergy Clin Immunol. 2016;138:615–619.e1.
Walter JE, Farmer JR, Foldvari Z, Torgerson TR, Cooper MA. Mechanism-based strategies for the management of autoimmunity and immune dysregulation in primary immunodeficiencies. J Allergy Clin Immunol Pract. 2016;4:1089–100.
McLean-Tooke A, Aldridge C, Waugh S, Spickett GP, Kay L. Methotrexate, rheumatoid arthritis and infection risk—what is the evidence? Rheumatology. 2009;48:867–71.
Downey C. Serious infection during etanercept, infliximab and adalimumab therapy for rheumatoid arthritis: a literature review. Int J Rheum Dis. 2016;19:536–50.
Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–602.
Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9:1911.
Xu L, Wang J, Liu Y, Xie L, Su B, Mou D, et al. CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med. 2019;381:1240–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SK and TI received honoraria from Bristol-Myers Squibb. TI received research funding from Ono pharmaceutical Co. The other authors declare no potential conflicts of interest.
Informed consent
All authors contributed to patient care and wrote the report. Informed consent was obtained from the patient for publication of this case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ureshino, H., Koarada, S., Kamachi, K. et al. Immune dysregulation syndrome with de novo CTLA4 germline mutation responsive to abatacept therapy. Int J Hematol 111, 897–902 (2020). https://doi.org/10.1007/s12185-020-02834-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-02834-9