Abstract
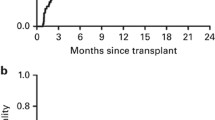
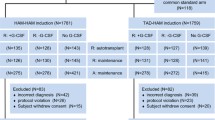
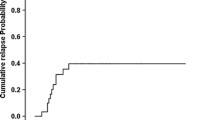
We prospectively compared outcomes of autologous stem cell transplantation (ASCT) versus high-dose cytarabine (HiDAC) consolidation as post-remission therapy for favorable- and intermediate-risk acute myelogenous leukemia (AML) in first complete remission (CR1). Two-hundred-forty patients under 65 years with AML-M1, M2, M4, or M5 subtypes were enrolled. After induction, 153 patients did not undergo randomization, while the remaining 87 who achieved CR1 were prospectively randomized to HiDAC (n = 45) or ASCT arm (n = 42). In the HiDAC arm, 43 patients completed three cycles of HiDAC, whereas in ASCT arm 22 patients completed two cycles of consolidation consisting of intermediate-dose cytarabine plus mitoxantrone or etoposide followed by ASCT. The three-year disease-free survival (DFS) rate was 41% in HiDAC and 55% in ASCT arm (p = 0.25). Three-year overall survival (OS) rates were 77 and 68% (p = 0.67). Incidence of relapse was 54 and 41% (p = 0.22). There was no significant difference in nonrelapse mortality between two arms (p = 0.88). Patients in the ASCT arm tended to have higher DFS rates and lower relapse rates than patients in HiDAC; however, there was no significant improvement in OS in patients with favorable- and intermediate-risk AML in CR1. Patients with AML are not benefited by the intensified chemotherapy represented by ASCT.


Similar content being viewed by others
References
Mrozek K, Marcucci G, Nicolet D, Maharry KS, Becker H, Whitman SP, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30(36):4515–23.
Schlenk RF. Post-remission therapy for acute myeloid leukemia. Haematologica. 2014;99(11):1663–70.
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–83.
Bloomfield CD, Lawrence D, Byrd JC, Carroll A, Pettenati MJ, Tantravahi R, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58(18):4173–9.
O’Donnel MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Bhatt V, et al. NCCN clinical practice guidelines in oncology. AML. NCCN guidelines version 3. 2017. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Accessed 13 Dec 2017.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47.
Miyawaki S. JSH guideline for tumors of hematopoietic and lymphoid tissues: leukemia 1. Acute myeloid leukemia (AML). Int J Hematol. 2017;106(3):310–25.
Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–61.
Burnett AK, Wheatley K, Goldstone AH, Stevens RF, Hann IM, Rees JH, et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol. 2002;118(2):385–400.
Zittoun RA, Mandelli F, Willemze R, de Witte T, Labar B, Resegotti L, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995;332(4):217–23.
Suciu S, Mandelli F, de Witte T, Zittoun R, Gallo E, Labar B, et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood. 2003;102(4):1232–40.
Vellenga E, van Putten W, Ossenkoppele GJ, Verdonck LF, Theobald M, Cornelissen JJ, et al. Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood. 2011;118(23):6037–42.
Zuckerman T, Beyar-Katz O, Rowe JM. Should autotransplantation in acute myeloid leukemia in first complete remission be revisited? Curr Opin Hematol. 2016;23(2):88–94.
Gorin NC, Labopin M, Frassoni F, Milpied N, Attal M, Blaise D, et al. Identical outcome after autologous or allogeneic genoidentical hematopoietic stem-cell transplantation in first remission of acute myelocytic leukemia carrying inversion 16 or t(8;21): a retrospective study from the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26(19):3183–8.
Kuwatsuka Y, Miyamura K, Suzuki R, Kasai M, Maruta A, Ogawa H, et al. Hematopoietic stem cell transplantation for core binding factor acute myeloid leukemia: t(8;21) and inv(16) represent different clinical outcomes. Blood. 2009;113(9):2096–103.
Usuki K, Kurosawa S, Uchida N, Yakushiji K, Waki F, Matsuishi E, et al. Comparison of autologous hematopoietic cell transplantation and chemotherapy as postremission treatment in non-M3 acute myeloid leukemia in first complete remission. Clin Lymphoma Myeloma Leuk. 2012;12(6):444–51.
Saraceni F, Bruno B, Lemoli RM, Meloni G, Arcese W, Falda M, et al. Autologous stem cell transplantation is still a valid option in good- and intermediate-risk AML: a GITMO survey on 809 patients autografted in first complete remission. Bone Marrow Transpl. 2017;52(1):163–6.
Harada M, Akashi K, Hayashi S, Eto T, Takamatsu Y, Teshima T, et al. Granulocyte colony-stimulating factor-combined marrow-ablative chemotherapy and autologous blood cell transplantation for the treatment of patients with acute myelogenous leukemia in first remission. The Fukouka Bone Marrow Transplant Group. Int J Hematol. 1997;66(3):297–301.
Gondo H, Harada M, Miyamoto T, Takenaka K, Tanimoto K, Mizuno S, et al. Autologous peripheral blood stem cell transplantation for acute myelogenous leukemia. Bone Marrow Transpl. 1997;20(10):821–6.
Eto T, Takase K, Miyamoto T, Ohno Y, Kamimura T, Nagafuji K, et al. Autologous peripheral blood stem cell transplantation with granulocyte colony-stimulating factor combined conditioning regimen as a postremission therapy for acute myelogenous leukemia in first complete remission. Int J Hematol. 2013;98(2):186–96.
Yoshimoto G, Nagafuji K, Miyamoto T, Kinukawa N, Takase K, Eto T, et al. FLT3 mutations in normal karyotype acute myeloid leukemia in first complete remission treated with autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2005;36(11):977–83.
Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhauser M, Juliusson G, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9(10):579–90.
Paschka P, Dohner K. Core-binding factor acute myeloid leukemia: can we improve on HiDAC consolidation? Hematol Am Soc Hematol Educ Program. 2013;2013:209–19.
Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P, et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood. 2013;121(12):2213–23.
Boissel N, Leroy H, Brethon B, Philippe N, de Botton S, Auvrignon A, et al. Incidence and prognostic impact of c-Kit, FLT3, and Ras gene mutations in core binding factor acute myeloid leukemia (CBF-AML). Leukemia. 2006;20(6):965–70.
Cairoli R, Beghini A, Grillo G, Nadali G, Elice F, Ripamonti CB, et al. Prognostic impact of c-KIT mutations in core binding factor leukemias: an Italian retrospective study. Blood. 2006;107(9):3463–8.
Shima T, Miyamoto T, Kikushige Y, Yuda J, Tochigi T, Yoshimoto G, et al. The ordered acquisition of Class II and Class I mutations directs formation of human t(8;21) acute myelogenous leukemia stem cell. Exp Hematol. 2014;42(11):955 e1–5–965 e1–5.
Boissel N, Renneville A, Leguay T, Lefebvre PC, Recher C, Lecerf T, et al. Dasatinib in high-risk core binding factor acute myeloid leukemia in first complete remission: a French Acute Myeloid Leukemia Intergroup trial. Haematologica. 2015;100(6):780–5.
Hospital MA, Prebet T, Bertoli S, Thomas X, Tavernier E, Braun T, et al. Core-binding factor acute myeloid leukemia in first relapse: a retrospective study from the French AML Intergroup. Blood. 2014;124(8):1312–9.
Ustun C, Marcucci G. Emerging diagnostic and therapeutic approaches in core binding factor acute myeloid leukaemia. Curr Opin Hematol. 2015;22(2):85–91.
Burnett AK, Russell NH, Hills RK, Kell J, Freeman S, Kjeldsen L, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30(32):3924–31.
Odenike OM, Alkan S, Sher D, Godwin JE, Huo D, Brandt SJ, et al. Histone deacetylase inhibitor romidepsin has differential activity in core binding factor acute myeloid leukemia. Clin Cancer Res. 2008;14(21):7095–101.
Bots M, Verbrugge I, Martin BP, Salmon JM, Ghisi M, Baker A, et al. Differentiation therapy for the treatment of t(8, 21) acute myeloid leukemia using histone deacetylase inhibitors. Blood. 2014;123(9):1341–52.
Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol. 2017;18(8):1061–75.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–21.
Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer. 2011;2(2):95–107.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–64.
Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722–31.
The Leukemia & Lymphoma Society. Study seeks new AML therapies. Cancer Discov. 2016;6(12):1297–8.
Hanekamp D, Cloos J, Schuurhuis GJ. Leukemic stem cells: identification and clinical application. Int J Hematol. 2017;105(5):549–57.
Acknowledgements
We thank the medical and nursing staff working on the JSCT for providing patient information. This work was supported by a Grant from the Regional Medicine Research Foundation (Tochigi, Japan). This work was also supported by a Grant-in-Aid for Scientific Research (no. 16H05340 to T.M.) and Grant-in-Aid for Scientific Research on Innovative Areas “Stem Cell Aging and Disease” (no. 25115002 to T.M.).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. T.M., K.N., and M.H. designed this study, collected clinical information, and wrote the manuscript. S.T, K.T, G.Y, S.Y, H.H, K.K, K.O, T.A., H.I, H.H, T.T, and K.A contributed to the collection of clinical information. All authors read and approved the final manuscript.
About this article
Cite this article
Miyamoto, T., Nagafuji, K., Fujisaki, T. et al. Prospective randomization of post-remission therapy comparing autologous peripheral blood stem cell transplantation versus high-dose cytarabine consolidation for acute myelogenous leukemia in first remission. Int J Hematol 107, 468–477 (2018). https://doi.org/10.1007/s12185-017-2389-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-017-2389-8