Abstract
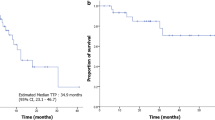
As part of an effort to develop a more effective and safe treatment for relapsed or refractory non-Hodgkin’s lymphoma (NHL), we conducted a phase II study of the oxaliplatin, etoposide, and ifosfamide (IFETOx) regimen. Patients with relapsed or refractory NHL and a performance status of 0–2 were eligible. The IFETOx consisted of etoposide at 100 mg/m2 on days 1–3, oxaliplatin at 130 mg/m2 on day 2, and ifosfamide 5,000 mg/m2 on day 2, every 21 days. The primary endpoint was the overall response rate (ORR) for IFETOx regimen. A total of 23 eligible patients were enrolled. The median age was 58 years (range 19–76 years), and the male-to-female ratio was 15:8. The disease status was as follows: 15 patients had relapsed and 8 patients were refractory to treatment. The ORR for IFETOx chemotherapy was 65.2 %. In the 15 patients who responded to the protocol treatment, five underwent hematopoietic stem cell transplantation. The 2-year probability of progression-free survival and overall survival rates were 51.4 and 56.1 %, respectively. Grade 3/4 neutropenia was observed in 73.9 % of the patients. No significant renal impairment was observed. In conclusion, IFETOx chemotherapy shows a tolerable toxicity profile and efficacy as a salvage treatment regimen for relapsed or refractory NHL.

Similar content being viewed by others
References
Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002–6.
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–5.
Sym SJ, Lee DH, Kang HJ, Nam SH, Kim HY, Kim SJ, et al. A multicenter phase II trial of etoposide, methylprednisolone, high-dose cytarabine, and oxaliplatin for patients with primary refractory/relapsed aggressive non-Hodgkin’s lymphoma. Cancer Chemother Pharmacol. 2009;64:27–33.
Velasquez WS, Cabanillas F, Salvador P, McLaughlin P, Fridrik M, Tucker S, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high-dose Ara-C and dexamethasone (DHAP). Blood. 1988;71:117–22.
Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, et al. ESHAP–an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12:1169–76.
Moskowitz CH, Bertino JR, Glassman JR, Hedrick EE, Hunte S, Coady-Lyons N, et al. Ifosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17:3776–85.
Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol. 1998;9:1053–71.
Germann N, Brienza S, Rotarski M, Emile JF, Di Palma M, Musset M, et al. Preliminary results on the activity of oxaliplatin (L-OHP) in refractory/recurrent non-Hodgkin’s lymphoma patients. Ann Oncol. 1999;10:351–4.
Machover D, Delmas-Marsalet B, Misra SC, Gumus Y, Goldschmidt E, Schilf A, et al. Dexamethasone, high-dose cytarabine, and oxaliplatin (DHAOx) as salvage treatment for patients with initially refractory or relapsed non-Hodgkin’s lymphoma. Ann Oncol. 2001;12:1439–43.
Chau I, Webb A, Cunningham D, Hill M, Rao S, Ageli S, et al. An oxaliplatin-based chemotherapy in patients with relapsed or refractory intermediate and high-grade non-Hodgkin’s lymphoma. Br J Haematol. 2001;115:786–92.
Oki Y, McLaughlin P, Pro B, Hagemeister FB, Bleyer A, Loyer E, et al. Phase II study of oxaliplatin in patients with recurrent or refractory non-Hodgkin lymphoma. Cancer. 2005;104:781–7.
Park BB, Kim WS, Eom HS, Kim JS, Lee YY, Oh SJ, et al. Salvage therapy with gemcitabine, ifosfamide, dexamethasone, and oxaliplatin (GIDOX) for B-cell non-Hodgkin’s lymphoma: a consortium for improving survival of lymphoma (CISL) trial. Invest New Drugs. 2011;29:154–60.
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–49.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244.
Rixe O, Ortuzar W, Alvarez M, Parker R, Reed E, Paull K, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute’s Anticancer Drug Screen panel. Biochem Pharmacol. 1996;52:1855–65.
Machover D, Diaz-Rubio E, de Gramont A, Schilf A, Gastiaburu JJ, Brienza S, et al. Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann Oncol. 1996;7:95–8.
Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A, et al. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347:353–7.
Conflict of interest
This study was supported by a grant of the Korean Heath Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A070001).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kim, SK., Song, MK., Chung, J.S. et al. Phase II study of ifosfamide, etoposide, and oxaliplatin (IFETOx) chemotherapy for relapsed or refractory non-Hodgkin’s lymphoma. Int J Hematol 98, 543–548 (2013). https://doi.org/10.1007/s12185-013-1440-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-013-1440-7