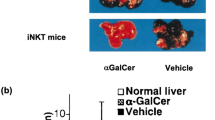
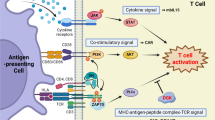
Abstract
Invariant natural killer T (iNKT) cells are characterized by the expression of an invariant Vα14–Jα18 paired with Vβ8/7/2 in mice, and Vα24–Jα18 with Vβ11 in humans, that recognizes glycolipids, such as α-galactosylceramide (α-GalCer), presented on the MHC class I-like molecule, CD1d. iNKT cells act as innate T lymphocytes and serve as a bridge between the innate and acquired immune systems. iNKT cells augment anti-tumor responses by producing IFN-γ, which acts on NK cells to eliminate MHC-non-restricted (MHC−) target tumor cells, and on CD8+ cytotoxic T lymphocytes to directly kill MHC-restricted (MHC+) tumor cells. Thus, when iNKT cells are activated by α-GalCer-pulsed dendritic cells, both MHC− and MHC+ tumor cells can be effectively eliminated. Both of these tumor cell types are simultaneously present in cancer patients, and at present iNKT cells are only the cell type capable of eliminating them. Based on these findings, we have developed iNKT cell-targeted adjuvant immunotherapies with strong anti-tumor activity in humans. However, two-thirds of patients were ineligible for this therapy due to the limited numbers of iNKT cells in their bodies. In order to overcome the problem in cancer patients, we successfully established a method to generate iNKT cells with adjuvant activity from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). In this review, we would like to outline the clinical potential for iNKT cells derived from ESCs and iPSCs for cancer immunotherapy, and the technical hurdles that must be overcome if we achieve effective ESC/iPSC-mediated cancer therapies.


Similar content being viewed by others
References
Taniguchi M, Harada M, Kojo S, et al. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513.
Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J Exp Med. 1994;180:1097–106.
Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–6.
Bendelac A, Lantz O, Quimby ME, et al. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–5.
Exley M, Garcia J, Balk SP, et al. Requirements for CD1d recognition by human invariant Vα24+CD4−CD8− T cells. J Exp Med. 1997;186:109–20.
Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of Valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9.
Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6.
Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336.
Taniguchi M, Seino K, Nakayama T. The NKT cell system: bridging innate and acquired immunity. Nat Immunol. 2003;4:1164–5.
Michel ML, Keller AC, Paget C, et al. Identification of an IL-17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001.
Tomura M, Yu WG, Ahn HJ, et al. A novel function of Vα14+CD4+ NKT cells: stimulation of IL-12 production by antigen-presenting cells in the innate immune system. J Immunol. 1999;163:93–101.
Kitamura H, Iwakabe K, Yahata T, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–8.
Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, et al. α-Galactosylceramide-activated Vα14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97:8461–6.
Trobonjaca Z, Leithäuser F, Möller P, et al. Activating immunity in the liver. I. Liver dendritic cells (but not hepatocytes) are potent activators of IFN-γ release by liver NKT cells. J Immunol. 2001;167:1413–22.
Stober D, Jomantaite I, Schirmbeck R, et al. NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo. J Immunol. 2003;170:2540–8.
Hermans IF, Silk JD, Gileadi U, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–7.
Fujii S, Shimizu K, Smith C, et al. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–79.
Fujii S, Liu K, Smith C, et al. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–18.
Fujii S, Shimizu K, Hemmi H, et al. Innate Vα14+ natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–98.
Khong HT, Restifo NP. Natural selection of tumor variants in the generation of tumor escape phenotypes. Nat Immunol. 2002;3:999–1005.
Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27.
Toura I, Kawano T, Akutsu Y, et al. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with a-galactosylceramide. J Immunol. 1999;163:2387–91.
Nieda M, Okai M, Tazbirkova A, et al. Therapeutic activation of Vα24+Vβ11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–9.
Chang DH, Osman K, Connolly J, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–17.
Ishikawa A, Motohashi S, Ishikawa E, et al. A phase I study of α-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–7.
Motohashi S, Ishikawa A, Ishikawa E, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12:6079–86.
Motohashi S, Nakayama T. Clinical applications of natural killer T cell-based immunotherapy for cancer. Cancer Sci. 2008;99:638–45.
Motohashi S, Nagato K, Kunii N, et al. A phase I–II study of α-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182:2492–501.
Nakayama N, Fang I, Elliott G. Natural killer and B-lymphoid potential in CD34+ cells derived from embryonic stem cells differentiated in the presence of vascular endothelial growth factor. Blood. 1998;91:2283–95.
Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–101.
Cho SK, Webber TD, Carlyle JR, et al. Functional characterization of B lymphocytes generated in vitro from embryonic stem cells. Proc Natl Acad Sci USA. 1999;96:9797–802.
de Pooter RF, Cho SK, Carlyle JR, et al. In vitro generation of T lymphocytes from embryonic stem cell-derived prehematopoietic progenitors. Blood. 2003;102:1649–53.
Schmitt TM, de Pooter RF, Gronski MA, et al. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5:410–7.
Hochedlinger K, Jaenisch R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature. 2002;415:1035–8.
Inoue K, Wakao H, Ogonuki N, et al. Generation of cloned mice by direct nuclear transfer from natural killer T cells. Curr Biol. 2005;15:1114–8.
Watarai H, Rybouchkin A, Hongo N, et al. Generation of functional NKT cells in vitro from embryonic stem cells bearing rearranged invariant Vα14–Jα18 TCRα gene. Blood. 2010;115:230–7.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7.
Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–24.
Hong H, Takahashi K, Ichisaka T, et al. Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature. 2009;460:1132–5.
Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–56.
Benlagha K, Kyin T, Beavis A, et al. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–5.
Pellicci DG, Hammond KJ, Uldrich AP, et al. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. J. J Exp Med. 2002;195:835–44.
Hanna J, Markoulaki S, Schorderet P, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–64.
Brown M, Rondon E, Rajesh D, et al. Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PLoS One. 2010;5:e11373.
Seki T, Yuasa S, Oda M, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–4.
Loh YH, Hartung O, Li H, et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7:15–9.
Staerk J, Dawlaty MM, Gao Q, et al. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7:20–4.
Watarai H, Nakagawa R, Omori-Miyake M, et al. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protoc. 2008;3:70–8.
Watarai H, Fujii S, Yamada D, et al. Murine induced pluripotent stem cells can be derived from and differentiate into natural killer T cells. J Clin Invest. 2010;120:2610–8.
Seki T, Yuasa S, Oda M, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–4.
Soldner F, Laganiere J, Cheng AW, et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146:318–31.
Chen F, Pruett-Miller SM, Huang Y, et al. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nat Methods. 2011;8:753–5.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Watarai, H., Yamada, D., Fujii, Si. et al. Induced pluripotency as a potential path towards iNKT cell-mediated cancer immunotherapy. Int J Hematol 95, 624–631 (2012). https://doi.org/10.1007/s12185-012-1091-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-012-1091-0