Abstract
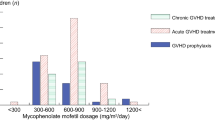
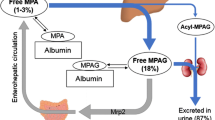
Mycophenolate mofetil (MMF) has been widely used for prophylaxis against graft-versus-host disease (GVHD) following allogeneic hematopoietic stem cell transplantation (allo-SCT). However, no clear advantage over methotrexate has been reported, other than reduced incidence of mucositis. We speculated that the wide inter-individual variation of plasma mycophenolic acid (MPA) levels veiled the benefits of MMF. Data from 36 unrelated allogeneic bone marrow (allo-BMT) and cord blood transplantation (CBT) were analyzed retrospectively based on MPA area under the curve (AUC0–24h). In allo-BMT, high AUC0–24h (>30 μg h/ml) resulted in no incidence of grade II–IV acute/extensive chronic GVHD and tended to show higher overall and disease-free survival, lower relapse rates, and non-relapse mortality. In CBT, AUC0–24h less than 30 μg h/ml was sufficient for low incidence of acute/chronic GVHD and high survival. Strong correlation between AUC0–24h and C2h, plasma MPA concentration at 2 h after administration was observed. Single point assessment of C2h was shown to provide a useful surrogate of AUC0–24h to predict GVHD incidence. The results of this study suggest that individualized MMF dosing in a donor source-dependent fashion may be important for maximizing the benefit of MMF in allo-SCT.




Similar content being viewed by others
References
Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood. 1989;73:1729–34.
Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–35.
Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation. 1995;60:225–32.
Maris MB, Sandmaier BM, Storer BE, Maloney DG, Shizuru JA, Agura E, et al. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after nonmyeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biol Blood Marrow Transplant. 2006;12:454–65.
Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–70.
Nieto Y, Patton N, Hawkins T, Spearing R, Bearman SI, Jones RB, et al. Tacrolimus and mycophenolate mofetil after nonmyeloablative matched-sibling donor allogeneic stem-cell transplantations conditioned with fludarabine and low-dose total body irradiation. Biol Blood Marrow Transplant. 2006;12:217–25.
Kawamori Y, Yakushijin K, Okamura A, Nishikawa S, Minagawa K, Shimoyama M, et al. Successful engraftment in reduced-intensity cord blood transplantation (CBT) as a salvage therapy for graft failure after primary CBT in adults. Transplantation. 2007;83:1281–2.
Jacobson P, Rogosheske J, Barker JN, Green K, Ng J, Weisdorf D, et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther. 2005;78:486–500.
Perez-Simon JA, Martino R, Caballero D, Valcarcel D, Rebollo N, de la Camara R, et al. Reduced-intensity conditioning allogeneic transplantation from unrelated donors: evaluation of mycophenolate mofetil plus cyclosporin A as graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2008;14:664–71.
Bolwell B, Sobecks R, Pohlman B, Andresen S, Rybicki L, Kuczkowski E, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;34:621–5.
Osunkwo I, Bessmertny O, Harrison L, Cheung YK, Van de Ven C, del Toro G, et al. A pilot study of tacrolimus and mycophenolate mofetil graft-versus-host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant. 2004;10:246–58.
Nash RA, Johnston L, Parker P, McCune JS, Storer B, Slattery JT, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:495–505.
Giaccone L, McCune JS, Maris MB, Gooley TA, Sandmaier BM, Slattery JT, et al. Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation. Blood. 2005;106:4381–8.
Neumann F, Graef T, Tapprich C, Vaupel M, Steidl U, Germing U, et al. Cyclosporine A and mycophenolate mofetil vs cyclosporine A and methotrexate for graft-versus-host disease prophylaxis after stem cell transplantation from HLA-identical siblings. Bone Marrow Transplant. 2005;35:1089–93.
Okamura A, Yamamori M, Shimoyama M, Kawano Y, Kawano H, Kawamori Y, et al. Pharmacokinetics-based optimal dose-exploration of mycophenolate mofetil in allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2008;88:104–10.
Okamura A, Shimoyama M, Ishii S, Wakahashi K, Asada N, Kawano H, et al. Delayed neutrophil engraftment in cord blood transplantation with intensive administration of mycophenolate mofetil for GVHD prophylaxis. Bone Marrow Transplant. 2011;46:148–9.
Pinana JL, Valcarcel D, Fernandez-Aviles F, Martino R, Rovira M, Barba P, et al. MTX or mycophenolate mofetil with CsA as GVHD prophylaxis after reduced-intensity conditioning PBSCT from HLA-identical siblings. Bone Marrow Transplant. 2010;45:1449–56.
Nishikawa S, Okamura A, Yamamori M, Minagawa K, Kawamori Y, Kawano Y, et al. Extended mycophenolate mofetil administration beyond day 30 in allogeneic hematopoietic stem cell transplantation as preemptive therapy for severe graft-versus-host disease. Transplant Proc. 2009;41:3873–6.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17.
van Gelder T, Le Meur Y, Shaw LM, Oellerich M, DeNofrio D, Holt C, et al. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit. 2006;28:145–54.
MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113:2410–5.
Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I. Unadjusted analysis. Bone Marrow Transplant. 2001;28:909–15.
Haentzschel I, Freiberg-Richter J, Platzbecker U, Kiani A, Schetelig J, Illmer T, et al. Targeting mycophenolate mofetil for graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2008;42:113–20.
de Winter BC, Mathot RA, Sombogaard F, Neumann I, van Hest RM, Doorduijn JK, et al. Differences in clearance of mycophenolic acid among renal transplant recipients, hematopoietic stem cell transplant recipients, and patients with autoimmune disease. Ther Drug Monit. 2010;32:606–14.
Acknowledgments
This work was supported, in part, by the Grants-in-Aid for Scientific Research from the Ministry of Health, Welfare, and Labor in Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wakahashi, K., Yamamori, M., Minagawa, K. et al. Pharmacokinetics-based optimal dose prediction of donor source-dependent response to mycophenolate mofetil in unrelated hematopoietic cell transplantation. Int J Hematol 94, 193–202 (2011). https://doi.org/10.1007/s12185-011-0888-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-011-0888-6