Abstract
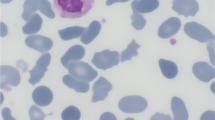
The implantation of a prosthetic heart valve (HVP) in patients with hereditary spherocytosis (HS) and hereditary elliptocytosis (HE) is rare, and the changes in the structure and deformability of erythrocytes that follow implantation in these patients have been poorly described. In the present study, the erythrocytes in HS and HE patients with mechanical HVP were compared to the erythrocytes in patients with only congenital membrane defects, in terms of biochemical modifications and rheological behaviour. Integral and cytoskeletal erythrocyte membrane proteins were studied, and blood viscosity (shear rate/shear stress ratio), aggregation ratio [η(1 s−1)/η(200 s−1)], and red cell visco-elasticity were determined. Valve replacement with a mechanical prosthesis worsened anaemia and resulted in a change in haemolysis, from sub-clinical to evident. The rheological investigation of erythrocytes from HS patients confirmed the characteristic increased viscosity and aggregation ratio and the decreased deformability. The rheological behaviour of erythrocytes from patients with HVP showed a decrease in viscosity and an increase in elastic modulus. In these patients, the prosthesis seems to have induced traumatic damage to the erythrocyte membrane, leading to fragmentation and lysis, which in turn modified rheological parameters. The biochemical and rheological investigation allowed us to understand the clinical and haematological pictures of the patients and to describe the role played by different factors in haemolytic anaemia.




Similar content being viewed by others
References
Lux SE, Palek J. Disorders of the red cell membrane. In: Handin RI, Lux SE, Stossel TO, editors. Principles and practise of Haematology. Philadelphia: Lippincott JB; 1995. p. 1701–818.
Gallagher PG, Jarolim P. Red blood cell membrane disorders, In: Hematology: basic principles and practice. Philadelphia, Pennsylvania: Elsevier; 2005. pp. 669–91.
Mohandas N, Chassis JA. Red blood cell deformability, membrane material properties and properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin Hematol. 1993;30:171–92.
Kuypers FA. Red cell membrane damage. J Heart Valve Dis. 1998;7:387–95.
Stuart J, Ellory JC. Rheological consequences of erythrocyte dehydration. Br J Haematol. 1988;69:1–4. doi:10.1111/j.1365-2141.1988.tb07593.x.
Fujii M, Nakajima K, Sakamoto K, Kanai H. Orientation and deformation of erythrocytes in flowing blood. Ann N Y Acad Sci. 1999;873:245–61. doi:10.1111/j.1749-6632.1999.tb09473.x.
Waugh RE, Agre P. Reductions of erythrocyte membrane viscoelastic coefficients reflect spectrin deficiencies in hereditary spherocytosis. J Clin Invest. 1988;81:133–41. doi:10.1172/JCI113284.
Chabanel A, Sung KLP, Rapiejko J, et al. Viscoelastic properties of red cell membrane in hereditary elliptocytosis. Blood. 1989;73:592–5.
Stuart J, Nash GB. Red cell deformability and haematological disorders. Blood Rev. 1990;4:141–7. doi:10.1016/0268-960X(90)90041-P.
Xiuli A, Lecomte MC, Chasis JA, et al. Shear-response of the spectrin dimer-tetramer equilibrium in the red blood cell membrane. J Biol Chem. 2002;277:31796–800. doi:10.1074/jbc.M204567200.
Dobbe JG, Hardeman MR, Strekstra GJ, et al. Analyzing red blood cell-deformability distributions. Blood Cells Mol Dis. 2002;28:373–84. doi:10.1006/bcmd.2002.0528.
Maraj R, Jacobs LE, Ioli A, Kotker MN. Evaluation of hemolysis in patients with prosthetic heart valves. Clin Cardiol. 1998;21:387–92. doi:10.1109/ICCV.1998.710781.
Ismeno G, Renzulli A, Carozza A, et al. Intra vascular hemolysis after mitral and aortic valve replacement with different types of mechanical prostheses. Int J Cardiol. 1999;69:179–83. doi:10.1016/S0167-5273(99)00024-8.
Birnbaum D, Lackzkovics A, Heidt M, et al. Examination of hemolytic potential with the On-XR prosthetic heart valve. J Heart Valve Dis. 2000;9:142–5.
Mizuno T, Tsukiya T, Taenaka Y, et al. Ultrastructural alterations in red blood cell membranes exposed to shear stress. ASAIO J. 2002;48:668–70. doi:10.1097/00002480-200211000-00017.
Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003;29:435–50. doi:10.1055/s-2003-44551.
Kawahira Y, Kishimoto H, Iio M, et al. Open heart operation in young child with spherocytosis. Ann Thorac Surg. 1994;58:1166–8.
Yamagishi I, Sakurada T, Abe T. Cardiac surgery using only autologous blood for a patient with hereditary spherocytosis: a case report. Ann Thorac Cardiovasc Surg. 1998;4:294–7.
Grigioni M, Caprari P, Tarzia A, D’Avenio G. Prosthetic heart valves’ mechanical loading of red blood cells in patients with hereditary membrane defects. J Biomech. 2005;38:1557–65. doi:10.1016/j.jbiomech.2004.11.020.
International Committee for Standardization in Haematology. Recommended methods for red-cell enzyme analysis. Br J Haematol. 1977;35:331–40. doi:10.1111/j.1365-2141.1977.tb00589.x.
Beutler E. Adenosin thriphosphate (ATP), In: Beutler E editor, Red cell metabolism, New York, Grune and Stratton, 1971, 92–4.
Beutler E. Reduced glutathione (GSH), In: Beutler E ed., Red cell metabolism, New York, Grune and Stratton, 1971, 103–5.
Zanella A, Izzo I, Rebulla P, et al. Acidified glycerol lysis test: a screening test for spherocytosis. Br J Haematol. 1980;45:481–6. doi:10.1111/j.1365-2141.1980.tb07167.x.
Caprari P, Bozzi A, Malorni W, et al. Junctional sites of erythrocyte skeletal proteins are specific targets of tert-butylhydroperoxide oxidative damage. Chem Biol Interact. 1995;94:243–58. doi:10.1016/0009-2797(94)03339-A.
International Committee for Standardization in Haematology (Export panel on blood rheology). Guidelines for measurement of blood viscosity and erythrocyte deformability. Clin Hemorheol. 1986;6:439–53.
Martorana MC, Mojoli G, Cianciulli P, Tarzia A, Mannella E, Caprari P. Sickle cell anaemia: haemorheological aspects. Ann Ist Super Sanita. 2007;43:164–70.
Mokken FC, Kedaria M, Henny CP, et al. The clinical importance of erythrocyte deformability, a hemorheological parameter. Ann Hematol. 1992;64:113–22. doi:10.1007/BF01697397.
Giersiepen M, Wurzinger LJ, Opitz R, Reul H. Estimation of shear stress-related blood damage in heart valve prostheses—in vitro comparison of 25 aortic valves. Int J Artif Organs. 1990;13:300–6.
Steegers A, Paul R, Reul H, Rau G. Leakage flow at mechanical heart valve prostheses: improved washout or increased blood damage? J Heart Valve Dis. 1999;8:312–23.
Okumiya T, Ishikawa-Nishi M, Doi T, et al. Evaluation of intravascular haemolysis with erythrocyte creatine in patients with cardiac valve prostheses. Chest. 2004;125:2115–20. doi:10.1378/chest.125.6.2115.
Mitlyng BL, Chandrashekhar Y, Furne JK, et al. Use of breath carbon monoxide to measure the influence of prosthetic heart valves on erythrocyte survival. Am J Cardiol. 2006;97:1374–6. doi:10.1016/j.amjcard.2005.11.074.
Kameneva MV, Antaki JF. Mechanical trauma to blood. In: Baskurt OK, Harderman MR, Rampling MW, Meiselman HJ, editors, Handbook of hemorheology and hemodinamics, Amsterdam: IOS Press; 2007, pp. 206–27.
Ellis JT, Timothy MW, Yoganathan AP. Prosthesis-induced hemolysis: mechanism and quantification of shear stress. J Heart Valve Dis. 1998;7:376–86.
Zimmer R, Steegers A, Paul R, et al. Velocities, shear stresses and blood damage potential of the leakage jets of the Medtronic parallel TM bileaflet valve. Int J Artif Organs. 2000;23:41–8.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Caprari, P., Tarzia, A., Mojoli, G. et al. Hereditary spherocytosis and elliptocytosis associated with prosthetic heart valve replacement: rheological study of erythrocyte modifications. Int J Hematol 89, 285–293 (2009). https://doi.org/10.1007/s12185-009-0270-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-009-0270-0