Abstract
Purpose of Review
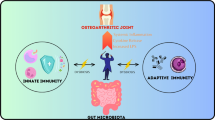
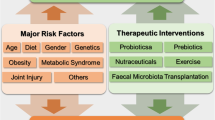
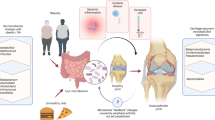
The importance of the gut microbiome has received increasing attention in recent years. New literature has revealed significant associations between gut health and various orthopedic disorders, as well as the potential for interventions targeting the gut microbiome to prevent disease and improve musculoskeletal outcomes. We provide a broad overview of available literature discussing the links between the gut microbiome and pathogenesis and management of orthopedic disorders.
Recent Findings
Human and animal models have characterized the associations between gut microbiome dysregulation and diseases of the joints, spine, nerves, and muscle, as well as the physiology of bone formation and fracture healing. Interventions such as probiotic supplementation and fecal transplant have shown some promise in ameliorating the symptoms or slowing the progression of these disorders.
Summary
We aim to aid discussions regarding optimization of patient outcomes in the field of orthopedic surgery by providing a narrative review of the available evidence-based literature involving gut microbiome dysregulation and its effects on orthopedic disease. In general, we believe that the gut microbiome is a viable target for interventions that can augment current management models and lead to significantly improved outcomes for patients under the care of orthopedic surgeons.
Similar content being viewed by others
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kong G, Zhang W, Zhang S, et al. The gut microbiota and metabolite profiles are altered in patients with spinal cord injury. Mol Brain 2023;16(1):26. https://doi.org/10.1186/s13041-023-01014-0 [published Online First: 20230220].
Singh V, Lee G, Son H, et al. Butyrate producers, “the sentinel of gut”: their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front Microbiol. 2022;13:1103836. https://doi.org/10.3389/fmicb.2022.1103836[publishedOnlineFirst:20230112].
Abdelhamid AG, El-Masry SS, El-Dougdoug NK. Probiotic Lactobacillus and Bifidobacterium strains possess safety characteristics, antiviral activities and host adherence factors revealed by genome mining. Epma j 2019;10(4):337-50. https://doi.org/10.1007/s13167-019-00184-z [published Online First: 20190905].
Nowak A, Paliwoda A, Błasiak J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: a review of mechanisms and therapeutic perspectives. Crit Rev Food Sci Nutr 2019;59(21):3456-67. https://doi.org/10.1080/10408398.2018.1494539 [published Online First: 20181016].
Sampaio-Maia B, Simões-Silva L, Pestana M, Araujo R, Soares-Silva IJ. The role of the gut microbiome on chronic kidney disease. Adv Appl Microbiol 2016;96:65-94. https://doi.org/10.1016/bs.aambs.2016.06.002 [published Online First: 20160718].
Paun A, Danska JS. Modulation of type 1 and type 2 diabetes risk by the intestinal microbiome. Pediatr Diabetes 2016;17(7):469-77. https://doi.org/10.1111/pedi.12424 [published Online First: 20160803].
Burcelin R. Gut microbiota and immune crosstalk in metabolic disease. Mol Metab 2016;5(9):771-81. https://doi.org/10.1016/j.molmet.2016.05.016 [published Online First: 20160606].
Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. https://doi.org/10.1038/nature11582.
Ponziani FR, Picca A, Marzetti E, et al. Characterization of the gut-liver-muscle axis in cirrhotic patients with sarcopenia. Liver Int 2021;41(6):1320-34. https://doi.org/10.1111/liv.14876 [published Online First: 20210325].
Guan Z, Luo L, Liu S, et al. The role of depletion of gut microbiota in osteoporosis and osteoarthritis: a narrative review. Front Endocrinol (Lausanne) 2022;13:847401. https://doi.org/10.3389/fendo.2022.847401 [published Online First: 20220328].
Picca A, Fanelli F, Calvani R, et al. Gut dysbiosis and muscle aging: searching for novel targets against sarcopenia. Mediators Inflamm 2018;2018:7026198. https://doi.org/10.1155/2018/7026198 [published Online First: 20180130].
Wei Y, Zhu W, Gong J, et al. Fecal microbiota transplantation improves the quality of life in patients with inflammatory bowel disease. Gastroenterol Res Pract 2015;2015:517597. https://doi.org/10.1155/2015/517597 [published Online First: 20150604].
Vaughn BP, Vatanen T, Allegretti JR, et al. Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease. Inflamm Bowel Dis. 2016;22(9):2182–90. https://doi.org/10.1097/mib.0000000000000893.
Huang Z, Chen J, Li B, et al. Faecal microbiota transplantation from metabolically compromised human donors accelerates osteoarthritis in mice. Ann Rheum Dis 2020;79(5):646-56. https://doi.org/10.1136/annrheumdis-2019-216471 [published Online First: 20200323].
Collins KH, Schwartz DJ, Lenz KL, Harris CA, Guilak F. Taxonomic changes in the gut microbiota are associated with cartilage damage independent of adiposity, high fat diet, and joint injury. Sci Rep. 2021;11(1):14560. https://doi.org/10.1038/s41598-021-94125-4.
Holub MN, Wahhab A, Rouse JR, et al. Peptidoglycan in osteoarthritis synovial tissue is associated with joint inflammation. Res Sq 2023. https://doi.org/10.21203/rs.3.rs-2842385/v1 [published Online First: 20230428].
Wei J, Zhang C, Zhang Y, et al. Association between gut microbiota and symptomatic hand osteoarthritis: data from the Xiangya Osteoarthritis Study. Arthritis Rheumatol 2021;73(9):1656-62. https://doi.org/10.1002/art.41729 [published Online First: 20210806].
Bonato A, Zenobi-Wong M, Barreto G, Huang Z. A systematic review of microbiome composition in osteoarthritis subjects. Osteoarthritis Cartilage 2022;30(6):786-801. https://doi.org/10.1016/j.joca.2021.12.006 [published Online First: 20211225]
• Liu S, Li G, Xu H, et al. “Cross-talk” between gut microbiome dysbiosis and osteoarthritis progression: a systematic review. Front Immunol 2023;14:1150572. https://doi.org/10.3389/fimmu.2023.1150572 [published Online First: 20230425]. This paper is of importance because it establishes that gut microbiome composition may not be generalizable across population.
Das M, Cronin O, Keohane DM, et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology (Oxford). 2019;58(12):2295–304. https://doi.org/10.1093/rheumatology/kez302.
Valido E, Bertolo A, Fränkl GP, et al. Systematic review of the changes in the microbiome following spinal cord injury: animal and human evidence. Spinal Cord. 2022;60(4):288–300. https://doi.org/10.1038/s41393-021-00737-y.
Cho KH, Na HS, Jhun J, et al. Lactobacillus (LA-1) and butyrate inhibit osteoarthritis by controlling autophagy and inflammatory cell death of chondrocytes. Front Immunol 2022;13:930511. https://doi.org/10.3389/fimmu.2022.930511 [published Online First: 20221017].
He J, Xu S, Zhang B, et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging (Albany NY) 2020;12(9):8583-604. https://doi.org/10.18632/aging.103168 [published Online First: 20200511].
Li C, Huang Q, Yang R, et al. Gut microbiota composition and bone mineral loss-epidemiologic evidence from individuals in Wuhan, China. Osteoporos Int 2019;30(5):1003-13. https://doi.org/10.1007/s00198-019-04855-5 [published Online First: 20190121].
Wang J, Wang Y, Gao W, et al. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ 2017;5:e3450. https://doi.org/10.7717/peerj.3450 [published Online First: 20170615].
Ma S, Qin J, Hao Y, Shi Y, Fu L. Structural and functional changes of gut microbiota in ovariectomized rats and their correlations with altered bone mass. Aging (Albany NY) 2020;12(11):10736-53. https://doi.org/10.18632/aging.103290 [published Online First: 20200602].
Ma S, Qin J, Hao Y, Fu L. Association of gut microbiota composition and function with an aged rat model of senile osteoporosis using 16S rRNA and metagenomic sequencing analysis. Aging (Albany NY) 2020;12(11):10795-808. https://doi.org/10.18632/aging.103293 [published Online First: 20200602].
Yuan Y, Yang J, Zhuge A, Li L, Ni S. Gut microbiota modulates osteoclast glutathione synthesis and mitochondrial biogenesis in mice subjected to ovariectomy. Cell Prolif 2022;55(3):e13194. https://doi.org/10.1111/cpr.13194 [published Online First: 20220126].
Ozaki D, Kubota R, Maeno T, Abdelhakim M, Hitosugi N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos Int 2021;32(1):145-56. https://doi.org/10.1007/s00198-020-05728-y [published Online First: 20201125].
Zhou J, Wang R, Zhao R, et al. Intermittent parathyroid hormone alters gut microbiota in ovariectomized osteoporotic rats. Orthop Surg 2022;14(9):2330-38. https://doi.org/10.1111/os.13419 [published Online First: 20220810].
Ma S, Wang N, Zhang P, Wu W, Fu L. Fecal microbiota transplantation mitigates bone loss by improving gut microbiome composition and gut barrier function in aged rats. PeerJ 2021;9:e12293. https://doi.org/10.7717/peerj.12293 [published Online First: 20211021].
•• Yu J, Cao G, Yuan S, Luo C, Yu J, Cai M. Probiotic supplements and bone health in postmenopausal women: a meta-analysis of randomised controlled trials. BMJ Open 2021;11(3):e041393. https://doi.org/10.1136/bmjopen-2020-041393 [published Online First: 20210302]. This paper is of major importance because the meta-analysis of randomized controlled trials provides very high-quality evidence in line with the goals of this review.
Morales MG, Olguín H, Di Capua G, Brandan E, Simon F, Cabello-Verrugio C. Endotoxin-induced skeletal muscle wasting is prevented by angiotensin-(1-7) through a p38 MAPK-dependent mechanism. Clin Sci (Lond) 2015;129(6):461-76. https://doi.org/10.1042/cs20140840 [published Online First: 20150519].
Zhou J, Liu B, Liang C, Li Y, Song YH. Cytokine signaling in skeletal muscle wasting. Trends Endocrinol Metab 2016;27(5):335-47. https://doi.org/10.1016/j.tem.2016.03.002 [published Online First: 20160326].
van Tongeren SP, Slaets JP, Harmsen HJ, Welling GW. Fecal microbiota composition and frailty. Appl Environ Microbiol. 2005;71(10):6438–42. https://doi.org/10.1128/aem.71.10.6438-6442.2005.
Petersen LM, Bautista EJ, Nguyen H, et al. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 2017;5(1):98. https://doi.org/10.1186/s40168-017-0320-4 [published Online First: 20170810].
Jackson MA, Jeffery IB, Beaumont M, et al. Signatures of early frailty in the gut microbiota. Genome Med 2016;8(1):8. https://doi.org/10.1186/s13073-016-0262-7 [published Online First: 20160129].
Dasarathy J, McCullough AJ, Dasarathy S. Sarcopenia in alcoholic liver disease: clinical and molecular advances. Alcohol Clin Exp Res 2017;41(8):1419-31. https://doi.org/10.1111/acer.13425 [published Online First: 20170711].
•• Huang WC, Lee MC, Lee CC, et al. Effect of Lactobacillus plantarum TWK10 on exercise physiological adaptation, performance, and body composition in healthy humans. Nutrients 2019;11(11). https://doi.org/10.3390/nu11112836 [published Online First: 20191119]. This paper is of major importance because randomized controlled trials provide high-quality evidence in line with the goals of this review.
•• Theou O, Jayanama K, Fernández-Garrido J, et al. Can a prebiotic formulation reduce frailty levels in older people? J Frailty Aging 2019;8(1):48-52. https://doi.org/10.14283/jfa.2018.39. This paper is of major importance because randomized controlled trials provide high-quality evidence in line with the goals of this review.
• Picca A, Ponziani FR, Calvani R, et al. Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: results from the BIOSPHERE study. Nutrients 2019;12(1). https://doi.org/10.3390/nu12010065 [published Online First: 20191226]. This paper is of importance because it is a large study investigating the associations between the gut microbiome and frailty, with broad implications for the geriatric population.
Khosravi A, Mazmanian SK. Disruption of the gut microbiome as a risk factor for microbial infections. Curr Opin Microbiol 2013;16(2):221-7. https://doi.org/10.1016/j.mib.2013.03.009 [published Online First: 20130415].
Khosravi A, Yáñez A, Price JG, et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15(3):374–81. https://doi.org/10.1016/j.chom.2014.02.006.
Chassaing B, Aitken JD, Gewirtz AT, Vijay-Kumar M. Gut microbiota drives metabolic disease in immunologically altered mice. Adv Immunol. 2012;116:93–112. https://doi.org/10.1016/b978-0-12-394300-2.00003-x.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. https://doi.org/10.1038/nature05414.
Scher JU, Littman DR, Abramson SB. Microbiome in inflammatory arthritis and human rheumatic diseases. Arthritis Rheumatol. 2016;68(1):35–45. https://doi.org/10.1002/art.39259.
Kane AV, Dinh DM, Ward HD. Childhood malnutrition and the intestinal microbiome. Pediatr Res 2015;77(1-2):256-62. https://doi.org/10.1038/pr.2014.179 [published Online First: 20141030].
Chisari E, Cho J, Wouthuyzen-Bakker M, Parvizi J. Periprosthetic joint infection and the Trojan Horse theory: examining the role of gut dysbiosis and epithelial integrity. J Arthroplasty 2022;37(7):1369-74. https://doi.org/10.1016/j.arth.2022.03.030 [published Online First: 20220314].
Hernandez CJ, Yang X, Ji G, et al. Disruption of the gut microbiome increases the risk of periprosthetic joint infection in mice. Clin Orthop Relat Res. 2019;477(11):2588–98. https://doi.org/10.1097/corr.0000000000000851.
Zhao X, Zhang Z, Wang Y, et al. Association of antibiotic alterations in gut microbiota with decreased osseointegration of an intramedullary nail in mice with and without osteomyelitis. Front Endocrinol (Lausanne) 2021;12:774257. https://doi.org/10.3389/fendo.2021.774257 [published Online First: 20211209].
Carson MD, Warner AJ, Hathaway-Schrader JD, et al. Minocycline-induced disruption of the intestinal FXR/FGF15 axis impairs osteogenesis in mice. JCI Insight 2023;8(1). https://doi.org/10.1172/jci.insight.160578 [published Online First: 20230110].
Luna M, Guss JD, Vasquez-Bolanos LS, et al. Components of the gut microbiome that influence bone tissue-level strength. J Bone Miner Res 2021;36(9):1823-34. https://doi.org/10.1002/jbmr.4341 [published Online First: 20210604].
Dar HY, Perrien DS, Pal S, et al. Callus γδ T cells and microbe-induced intestinal Th17 cells improve fracture healing in mice. J Clin Invest 2023;133(8). https://doi.org/10.1172/jci166577 [published Online First: 20230417].
Moran MM, Wilson BM, Li J, et al. The gut microbiota may be a novel pathogenic mechanism in loosening of orthopedic implants in rats. Faseb j 2020;34(11):14302-17. https://doi.org/10.1096/fj.202001364R [published Online First: 20200915].
Yao B, Cai Y, Wang W, et al. The effect of gut microbiota on the progression of intervertebral disc degeneration. Orthop Surg 2023;15(3):858-67. https://doi.org/10.1111/os.13626 [published Online First: 20230104].
Li W, Lai K, Chopra N, Zheng Z, Das A, Diwan AD. Gut-disc axis: a cause of intervertebral disc degeneration and low back pain? Eur Spine J. 2022;31(4):917–25. https://doi.org/10.1007/s00586-022-07152-8.
Rajasekaran S, Soundararajan DCR, Tangavel C, et al. Human intervertebral discs harbour a unique microbiome and dysbiosis determines health and disease. Eur Spine J 2020;29(7):1621-40. https://doi.org/10.1007/s00586-020-06446-z [published Online First: 20200514].
Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 2014;10(1):44-56. https://doi.org/10.1038/nrrheum.2013.160 [published Online First: 20131029].
Kigerl KA, Zane K, Adams K, Sullivan MB, Popovich PG. The spinal cord-gut-immune axis as a master regulator of health and neurological function after spinal cord injury. Exp Neurol 2020;323:113085. https://doi.org/10.1016/j.expneurol.2019.113085 [published Online First: 20191022].
Zhang W, Li Y, Sun T, et al. Superior cervical ganglionectomy alters gut microbiota in rats. Am J Transl Res 2022;14(3):2037–50 [published Online First: 20220315].
Schmidt EKA, Torres-Espin A, Raposo PJF, et al. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS One 2020;15(1):e0226128. https://doi.org/10.1371/journal.pone.0226128 [published Online First: 20200115].
Doelman A, Tigchelaar S, McConeghy B, et al. Characterization of the gut microbiome in a porcine model of thoracic spinal cord injury. BMC Genomics 2021;22(1):775. https://doi.org/10.1186/s12864-021-07979-3 [published Online First: 20211030].
Rodenhouse A, Talukder MAH, Lee JI, et al. Altered gut microbiota composition with antibiotic treatment impairs functional recovery after traumatic peripheral nerve crush injury in mice: effects of probiotics with butyrate producing bacteria. BMC Res Notes 2022;15(1):80. https://doi.org/10.1186/s13104-022-05967-8 [published Online First: 20220223].
He N, Shen G, Jin X, et al. Resveratrol suppresses microglial activation and promotes functional recovery of traumatic spinal cord via improving intestinal microbiota. Pharmacol Res 2022;183:106377. https://doi.org/10.1016/j.phrs.2022.106377 [published Online First: 20220801].
Author information
Authors and Affiliations
Contributions
DH, AG, JI, and MP did the manuscript research and writing. DH, AE, and WH edited the manuscript. SK and WH generated the idea for the manuscript. MP and DH prepared the manuscript for submission. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
Dr. Wellington Hsu is an advisory board member of Stryker, Medtronic, Asahi, and Bioventus. Dr. Adam Edelstein is a consultant for Depuy and Corin; he is also on the Editorial Board of Arthroplasty Today and serves on the AAHKS Publications Committee.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hiltzik, D.M., Goodwin, A.M., Kurapaty, S.S. et al. The Role of the Gut Microbiome in Orthopedic Surgery—a Narrative Review. Curr Rev Musculoskelet Med 17, 37–46 (2024). https://doi.org/10.1007/s12178-023-09878-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-023-09878-4