Abstract
Triglyceride-rich lipoproteins—very low-density lipoproteins (VLDL) and chylomicrons—are considered atherogenic. The postprandial state, during which plasma levels of these lipoproteins are increased, is characterized by an inflammatory response that also involves endothelia. Free fatty acids released from chylomicrons and VLDL near the endothelium may be responsible for this. However, recent studies on mice and human subjects have revealed that intestinal absorption of dietary fat promotes absorption of bacterial lipopolysaccharides (LPS), which are atherogenic. Absorbed LPS is transported on chylomicrons, which suggests that LPS could contribute to postprandial inflammation. Recent work suggests that the intestinal microflora may influence atherosclerosis by affecting energy harvest from the diet. The novel link between intestinal absorption of fat and of LPS suggests that the microflora could also affect atherosclerosis by providing proinflammatory material to the bloodstream. Unraveling the chylomicron-associated LPS absorption mechanism and identification of micro-organisms providing the LPS may be beneficial for the prevention of atherosclerosis.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wiedermann CJ, Kiechl S, Dunzendorfer S, et al.: Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck study. J Am Coll Cardiol 1999, 34:1975–1981.
Duffy D, Rader DJ: Update on strategies to increase HDL quantity and function. Nat Rev Cardiol 2009, 6:455–463.
Florentin M, Liberopoulos EN, Wierzbicki AS, Mikhailidis DP: Multiple actions of high-density lipoprotein. Curr Opin Cardiol 2008, 23:370–378.
Tabas I, Williams KJ, Boren J: Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 2007, 116:1832–1844.
Steinberg D, Parthasarathy S, Carew TE, et al.: Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989, 320:915–924.
Burdge GC, Calder PC: Plasma cytokine response during the postprandial period: a potential causal process in vascular disease? Br J Nutr 2005, 93:3–9.
van Oostrom AJ, Sijmonsma TP, Verseyden C, et al.: Postprandial recruitment of neutrophils may contribute to endothelial dysfunction. J Lipid Res 2003, 44:576–583.
Margioris AN: Fatty acids and postprandial inflammation. Curr Opin Clin Nutr Metab Care 2009, 12:129–137.
•• Beigneux AP, Davies BS, Gin P, et al.: Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab 2007, 5:279–291. This article identifies a novel factor within the endothelium that promotes lipolytic clearance of chylomicrons.
Higgins LJ, Rutledge JC: Inflammation associated with the postprandial lipolysis of triglyceride-rich lipoproteins by lipoprotein lipase. Curr Atheroscler Rep 2009, 11:199–205.
Chung BH, Hennig B, Cho BH, Darnell BE: Effect of the fat composition of a single meal on the composition and cytotoxic potencies of lipolytically-releasable free fatty acids in postprandial plasma Atherosclerosis 1998, 141:321–332.
Shi H, Kokoeva MV, Inouye K, et al.: TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006, 116:3015–3025.
Suganami T, Tanimoto-Koyama K, Nishida J, et al.: Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol 2007, 27:84–91.
Lee JY, Sohn KH, Rhee SH, Hwang D: Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 2001, 276:16683–16689.
• Erridge C, Samani NJ: Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol 2009, 29:1944–1949. This article argues against a direct role of FFAs in the activation of TLR4, suggesting that other factors associated with lipoproteins are involved.
Michelsen KS, Wong MH, Shah PK, et al.: Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein Proc Natl Acad Sci USA 2004, 101:10679–10684.
Kiechl S, Lorenz E, Reindl M, et al.: Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med 2002, 347:185–192.
Ameziane N, Beillat T, Verpillat P, et al.: Association of the Toll-like receptor 4 gene Asp299Gly polymorphism with acute coronary events. Arterioscler Thromb Vasc Biol 2003, 23:e61–e64.
Berg RD: The indigenous gastrointestinal microflora. Trends Microbiol 1996, 4:430–435.
Turnbaugh PJ, Ley RE, Mahowald MA, et al.: An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444:1027–1031.
Backhed F, Manchester JK, Semenkovich CF, Gordon JI: Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 2007, 104:979–984.
• Turnbaugh PJ, Hamady M, Yatsunenko T, et al.: A core gut microbiome in obese and lean twins. Nature 2009, 457:480–484. This article reveals considerable interindividual variation in the gut microbiome among humans, yet it argues for the presence of a core microbiome that is associated with obesity.
McGill HC Jr, McMahan CA, Herderick EE, et al.: Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation 2002, 105:2712–2718.
• Queenan K, Stewart M, Smith K, et al.: Concentrated oat beta-glucan, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomized controlled trial. Nutr J 2007, 6:6. The authors performed human studies to show that butyrate mediates LDL-lowering effects of high-fiber diets.
Wachtershauser A, Loitsch SM, Stein J: PPAR-gamma is selectively upregulated in Caco-2 cells by butyrate. Biochem Biophys Res Commun 2000, 272:380–385.
Neutra MR, Pringault E, Kraehenbuhl JP: Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol 1996, 14:275–300.
Rescigno M, Urbano M, Valzasina B, et al.: Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001, 2:361–367.
Macpherson AJ, Uhr T: Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004, 303:1662–1665.
Nadhazi Z, Takats A, Offenmuller K, Bertok L: Plasma endotoxin level of healthy donors. Acta Microbiol Immunol Hung 2002, 49:151–157.
Ravin HA, Rowley D, Jenkins C, Fine J: On the absorption of bacterial endotoxin from the gastro-intestinal tract of the normal and shocked animal. J Exp Med 1960, 112:783–792.
•• Cani PD, Amar J, Iglesias MA, et al.: Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56:1761–1772. Mice on high-fat diets absorb LPS from the gut. Infusion of similar levels of LPS in mice on low-fat diets led to a similar phenotype as mice on high-fat diets. Thus, gut LPS contributes to metabolic disorders linked with high-fat dieting.
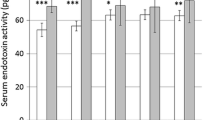
• Erridge C, Attina T, Spickett CM, Webb DJ: A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 2007, 86:1286–1292. The authors show, using human subjects, that postprandial lipid levels correlate with LPS levels, suggesting a link between LPS absorption and fat absorption.
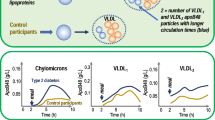
• Ghoshal S, Witta J, Zhong J, et al.: Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res 2009, 50:90–97. The authors show that chylomicron formation is required for the promotion of LPS absorption by dietary fat.
Kvietys PR, Specian RD, Grisham MB, Tso P: Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. Am J Physiol 1991, 261(3 Pt 1):G384–G391.
Harris HW, Grunfeld C, Feingold KR, et al.: Chylomicrons alter the fate of endotoxin, decreasing tumor necrosis factor release and preventing death. J Clin Invest 1993, 91:1028–1034.
Vreugdenhil AC, Snoek AM, Greve JM, Buurman WA: Lipopolysaccharide-binding protein is vectorially secreted and transported by cultured intestinal epithelial cells and is present in the intestinal mucus of mice. J Immunol 2000, 165:4561–4566.
Neal MD, Leaphart C, Levy R, et al.: Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol 2006, 176:3070–3079.
Hornef MW, Frisan T, Vandewalle A, et al.: Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med 2002, 195:559–570.
Luchoomun J, Hussain MM: Assembly and secretion of chylomicrons by differentiated Caco-2 cells. Nascent triglycerides and preformed phospholipids are preferentially used for lipoprotein assembly. J Biol Chem 1999, 274:19565–19572.
Read TE, Harris HW, Grunfeld C, et al.: The protective effect of serum lipoproteins against bacterial lipopolysaccharide. Eur Heart J 1993, 14(Suppl K):125–129.
Kasravi B, Lee DH, Lee JW, et al.: Chylomicron-bound LPS selectively inhibits the hepatocellular response to proinflammatory cytokines. J Surg Res 2008, 146:96–103.
Smythies LE, Sellers M, Clements RH, et al.: Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 2005, 115:66–75.
Acknowledgment
Dr. Erik Eckhardt is supported by the National Institutes of Health grant 1P20RR021954-01A2.
Disclosure
No potential conflict of interest relevant to this article was reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eckhardt, E. Metagenomics, Lipoproteins, and Cardiovascular Risk. Curr Cardio Risk Rep 4, 9–14 (2010). https://doi.org/10.1007/s12170-009-0067-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12170-009-0067-y