Abstract
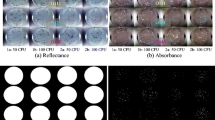
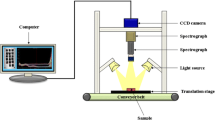
Salmonella causes illness in millions of people each year with severe cases resulting in death. Traditional detection methods are well established but are associated with disadvantages such as time or reoccurring sample cost. Hyperspectral microscope imaging (HMI) has shown potential as an early and rapid detection method, identifying bacteria based on spectral signatures unique to the microorganism. Bacteria undergo physiological changes when introduced to environmental stresses. Understanding how these physiological changes impact the resulting cellular spectra is critical to developing robust HMI methodologies for early and rapid detection of bacteria. Salmonella Enteritidis (SE) and Salmonella Typhimurium (ST) were incubated on plate agars of brilliant green sulfa, tryptic soy agar, xylose lysine deoxycholate, and xylose lysine tergitol 4, at incubation pH ranging from 4.7 to 8.3, and at incubation temperatures between 27 and 47 °C. All samples were incubated for 24 h. A principal component analysis (PCA) was performed for each experiment, with Mahalanobis distances (MD) calculated for each sample to its respective cluster’s center, quantifying intraclass variation. One-way ANOVA of MD values showed no significant difference for SE and ST grown on four agars (P > 0.05), or at varying incubation temperatures (P > 0.05). SE and ST incubated at five pH values showed significantly different spectra (P < 0.01). Results suggest that future HMI tools for identifying Salmonella can be developed regardless of the growth media or temperature but should take into consideration acidic growth environments.





Similar content being viewed by others
References
Abee T, Wouters JA (1999) Microbial stress response in minimal processing. Inter J Food Microbiol 50:65–91
Anderson J, Reynolds C, Ringelberg D, Edwards J, Foley K (2008) Differentiation of live-viable versus dead bacterial endospores by calibrated hyperspectral reflectance microscopy. J Microsc 232:130–136
Brereton R, Lloyd G (2016) Re-evaluating the role of the Mahlanobis distance measure. J Chemom 30:134–143
CDC (Centers for Disease Control and Prevention) (2019a) Salmonella: reports of selected Salmonella outbreak investigations. 25 January 2019. https://www.cdc.gov/salmonella/outbreaks.html Accessed 08.03.19
CDC (Centers for Disease Control and Prevention) (2019b) Foodborne diseases active surveillance network (FoodNet): FoodNet surveillance report. 16 July 2019. https://www.cdc.gov/foodnet/reports/prelim-data-intro-2018.html
Cotter PD, Hill C (2003) Surviving the acid test: response of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67:429–453
Eady M, Park B (2016) Classification of Salmonella enterica serotypes with selective bands using visible/NIR hyperspectral microscope images. J Microsc 263(1):10–19
Eady M, Park B, Choi S (2015) Rapid and early detection of Salmonella serotypes with hyperspectral microscopy and multivariate detection. J Food Prot 78:668–674
Eady MB, Park B, Yoon SC, Haidekker MA, Lawrence KC (2018) Methods for hyperspectral microscope calibration and spectra normalization from images of bacteria cells. Trans ASABE 61(2):437–448
Feng YZ, Sun DW (2013) Determination of total viable count (TVC) in chicken breast fillets by near-infrared hyperspectral imaging and spectroscopic transforms. Talanta 105:244–249
Kim H, Doh IJ, Sturgis J, Bhunia A, Robinson JP, Bae E (2016) Reflected scatterometry for noninvasive interrogation of bacterial colonies. J Biomed Opt 21:107004
Leyer GJ, Johnson EA (1993) Acid adaptation induces cross-protection against environmental stresses in Salmonella typhimurium. Appl Environ Microbiol 59(6):1842–1847
Li H, Wang H, D’Aoust JY, Maurer J (2013) Salmonella species. In: Doyle MP, Buchannon RL (eds) Food microbiology: fundamentals and frontiers 4th edn. ASM Press, Washington, DC, pp 225–262
Lu R, Park B (2015) Image and spectral analysis techniques: introduction. In: Park B, Lu R (eds) Hyperspectral imaging technology in food and agriculture. Springer, New York, pp 3–8
Moats W, Kinner J (1974) Factors effecting selectivity of brilliant green-phenol red agar for salmonellae. Appl Microbiol 27:118–123
Montville J, Matthews K (2013) Physiology, growth, and inhibition of microbes in foods. In: Doyle MP, Buchannon RL (eds) Food microbiology: fundamentals and frontiers 4th edn. ASM Press, Washington, DC, pp 3–18
Mullis K, Faloona F (1987) Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol 155:335–350
Park B, Eady M (2016) New applications of hyperspectral imaging for bacterial cell classification. Nir News 27:4–6
Park B, Seo Y, Yoon SC, Hinton A Jr, Windham W, Lawrence K (2015) Hyperspectral microscope imaging method to classify gram-positive and gram-negative foodborne pathogenic bacteria. Trans ASABE 58:5–16
Tang Y, Kim H, Singh AK, Aroonnual A, Bae E, Rajwa B, Fratamico PM, Bhunia A (2014) Light scattering sensor for direct identification of colonies of Escherichia coli O26, O45, O103, O145, and O157. PLoS One 9:1–15
Varmuza K, Filzmoser P (2007) Introduction to multivariate statistical analysis in chemometrics. CRC Press, Boca Raton
Wesche AM, Gurtler JB, Marks BP, Ryser ET (2009) Stress, sublethal injury, resuscitation, and virulence of bacterial foodborne pathogens. Journal of Food Protection 72(5):1121–1138
Williams P, Kammies TL, Gauws PA, Manley M (2019) Effects of colony age on near infrared images of foodborne bacteria. J Spectral Imaging 8a5:1–12
Windham W, Yoon SC, Ladely S, Haley J, Heitschmidt J, Lawrence K, Narrang N, Cray W (2013) Detection by hyperspectral imaging of shiga toxin-producing Escherichia coli serogroups O26, O45, O103, O111, O121, and O145 on rainbow agar. J Food Prot 76:1129–1136
Zhang L, Cleveland McEntire J, Newsome R, Wang H (2013) Antimicrobial resistance. In: Doyle MP, Buchannon RL (eds) Food microbiology: fundamentals and frontiers 4th edn. ASM Press, Washington, DC, pp 19–44
Acknowledgments
The authors would like to thank Drs. Nasreen Bano and Jing Chen, of the Quality and Safety Assessment Research Unit located at the U.S. National Poultry Research Center in Athens, GA, for their assistance with hyperspectral image collection and experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Matthew Eady declares that he has no conflict of interest. Bosoon Park declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 348 kb)
Rights and permissions
About this article
Cite this article
Eady, M., Park, B. The Influence of Environmental Growth Conditions on Salmonella Spectra Obtained from Hyperspectral Microscope Images. Food Anal. Methods 12, 2638–2646 (2019). https://doi.org/10.1007/s12161-019-01618-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01618-0