Abstract
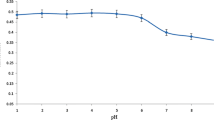
The presence of Cr(VI) in edible parts of vegetable plants might be representing a probable health risk to humans and animals. On the other hand, the presence of Cr(III) is beneficial for human health. A new method has been employed for the separation and pre-concentration of Cr(III)/Cr(VI) present in the aqueous extract (soup) of wild vegetables prior to determining with graphite furnace atomic absorption spectrometry. For the pre-concentration of Cr(III) and Cr(VI) in two sets of aqueous extract of each vegetable, complexing reagents, 8-hydroxyquinoline (oxine) and ammonium pyrrolidinedithiocarbamate (APDC), was added respectively. Then the tunable solvent solution (TSS) was added based on 1,8-diazabicyclo[5.4.0]undec-7-ene and decanol (1:1) in an aqueous system. The role of different variables for a proposed method such as the amount of ligands (oxine and APDC), pH of the solution, volume of TSS, purging time, and pressure of CO2 was investigated. The accuracy for the quantification of Cr(III) and Cr(VI) was determined by the standard addition method at optimized values of all factors, and > 98% of both species were obtained. The LOD and LOQ of Cr(III)/Cr(VI) were found to be 0.048/0.072 and 0.16/0.24 μg L−1, respectively. The data indicate that Cr(III) is found > 65% and Cr(VI) as < 35% in aqueous extract of all selected leafy vegetables, which is a good sign for public awareness to maintain human health by using these wild vegetables. These results will also be helpful to the researchers compiling the nutritional data sheet.

Graphical Abstract


Similar content being viewed by others
References
Akhtar A, Kazi TG, Afridi HI, Khan M, Bilal M, Khan N (2018) Application of modified cloud point extraction method for the chromium speciation in artificial saliva extracts of different snuff products. J Ind Eng Chem 59:320–327
Alauzun J, Besso E, Mehdi A, Reye C, Corriu RJ (2007) Reversible covalent chemistry of CO2: an opportunity for nano-structured hybrid organic–inorganic materials. Chem Mater 20:503–513
Andrle C, Jakubowski N, Broekaert J (1997) Speciation of chromium using reversed phase-high performance liquid chromatography coupled to different spectrometric detection methods. Spectrochim Acta B At Spectrosc 52:189–200
Anwar F, Latif S, Ashraf M, Gilani AH (2007) Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res 21:17–25
Baig JA, Hol A, Akdogan A, Kartal AA, Divrikli U, Kazi TG, Elci L (2012) A novel strategy for chromium speciation at ultra-trace level by microsample injection flame atomic absorption spectrophotometry. J Anal Atomic Spectrom 27:1509–1517
Barceló J, Poschenrieder C, Vázquez MD, Gunsé B (1993) Beneficial and toxic effects of chromium in plants: solution culture, pot and field studies. Studi Environ Sci 55:147–171
Barminas J, Charles M, Emmanuel D (1998) Mineral composition of non-conventional leafy vegetables. Plant Food Hum Nutr 53:29–36
Bulut VN, Ozdes D, Bekircan O, Gundogdu A, Duran C, Soylak M (2009) Carrier element-free coprecipitation (CEFC) method for the separation, preconcentration and speciation of chromium using an isatin derivative. Anal Chim Acta 632(1):35–41
da Conceicao Gomes MA, Hauser-Davis RA, Suzuki MS, Vitoria AP (2017) Plant chromium uptake and transport, physiological effects and recent advances in molecular investigations. Ecotoxicol Environ Saf 140:55–64
de Bezerra MA, Arruda MAZ, Ferreira SLC (2005) Cloud point extraction as a procedure of separation and pre-concentration for metal determination using spectroanalytical techniques: a review. Appl Spectrosc Rev 40:269–299
Fakankun O, Babayemi J, Utiaruk J (2013) Variations in the mineral composition and heavy metals content of Moringa oleifera. AJEST 7:371–379
Gikas P, Romanos P (2006) Effects of tri-valent (Cr (III)) and hexa-valent (Cr (VI)) chromium on the growth of activated sludge. J Hazard Mater 133:212–217
Gutowski KE, Maginn EJ (2008) Amine-functionalized task-specific ionic liquids: a mechanistic explanation for the dramatic increase in viscosity upon complexation with CO2 from molecular simulation. J Am Chem Soc 130:14690–14704
Heldebrant DJ, Yonker CR, Jessop PG, Phan L (2008) Organic liquid CO 2 capture agents with high gravimetric CO2 capacity. Energy Environ Sci 1:487–493
Hemmatkhah P, Bidari A, Jafarvand S, Hosseini MRM, Assadi Y (2009) Speciation of chromium in water samples using dispersive liquid–liquid microextraction and flame atomic absorption spectrometry. Microchimica Acta 166:69–75
Jakhar GS, Abro SA, Maher A, Qureshi R (2005) Weed communities of wheat crop under diverse edaphogropghy of district Khairpur, Pakistan. Pak J Bot 37:709
Jamali M, Kazi T, Arain MB, Afridi H, Jalbani N, Memon A (2007) Heavy metal contents of vegetables grown in soil, irrigated with mixtures of wastewater and sewage sludge in Pakistan, using ultrasonic-assisted pseudo-digestion. J Agron Crop Sci 193:218–228
Jessop PG, Heldebrant DJ, Li X, Eckert CA, Liotta CL (2005) Green chemistry: reversible nonpolar-to-polar solvent. Nature 436:1102
Jessop PG, Kozycz L, Rahami ZG, Schoenmakers D, Boyd AR, Wechsler D, Holland AM (2011) Tertiary amine solvents having switchable hydrophilicity. Green Chem 13:619–623
Kalidhasan S, Ganesh M, Sricharan S, Rajesh N (2009) Extractive separation and determination of chromium in tannery effluents and electroplating waste water using tribenzylamine as the extractant. J Hazard Mater 165:886–892
Kaszycki P, Gabrys H, Appenroth KJ, Jaglarz A, Sedziwy S, Walczak T, Koloczek H (2005) Exogenously applied sulphate as a tool to investigate transport and reduction of chromate in the duckweed Spirodela polyrhiza. Plant Cell Environ 28:260–268
Kazi TG, Afridi HI, Kazi N, Jamali MK, Arain MB, Jalbani N, Kandhro GA (2008) Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol Trace Elem Res 122:1–18
Khan M, Kazi TG, Afridi HI, Bilal M, Akhtar A, Ullah N, Khan S, Talpur S (2017) Application of ultrasonically modified cloud point extraction method for simultaneous enrichment of cadmium and lead in sera of different types of gallstone patients. Ultrason Sonochem 39:313–320
Kohlmeier M (2015) Nutrient metabolism: structures, functions, and genes. Elsevier/Academic Press, Amsterdam
Konstantinos K, Ennio R, Daniela R, Deok HM (2009) Reduction of Cr(VI) to Cr(III) in artificial, contaminated soil using ferrous sulfate heptahydrate and sodium thiosulfate. J Hazard Toxic Radioact 13:218–228
Kumar AR, Riyazuddin P (2009) The effect of Cr (III)-organic complexes on the determination of inorganic chromium species in groundwater by ammonium pyrrolidinedithiocarbamate–methylisobutylketone extraction procedure. Microchem J 92:145–149
Lasarte-Aragones G, Lucena R, Cardenas S, Valcarcel M (2015) Use of switchable solvents in the microextraction context. Talanta 131:645–649
Lopez-Luna J, Gonzalez-Chavez M, Esparza-Garcia F, Rodriguez-Vazquez R (2009) Toxicity assessment of soil amended with tannery sludge, trivalent chromium and hexavalent chromium, using wheat, oat and sorghum plants. J Hazard Mater 163:829–834
Mahapatra AK, Mishra S, Basak UC, Panda PC (2012) Nutrient analysis of some selected wild edible fruits of deciduous forests of India: an explorative study towards non conventional bio-nutrition. Adv J Food Sci Technol 4:15–21
Memon RA, Bhatti GR, Khalid S, Ahmed S (2014) Illustrated weed flora of cotton crop of Khairpur district, Sindh, Pakistan. Pak J Bot 46:5–12
Mercer SM, Robert T, Dixon DV, Chen CS, Ghoshouni Z, Harjani JR, Jahangiri S, Peslherbe GH, Jessop PG (2012) Design, synthesis, and solution behaviour of small polyamines as switchable water additives. Green Chem 14:832–839
Paleologos EK, Stalikas CD, Tzouwara-Karayanni SM, Pilidis GA, Karayannis MI (2000) Micelle-mediated methodology for speciation of chromium by flame atomic absorption spectrometry. J Anal Atomic Spectrom, 15:287–291
Pyrzynska K (2012) Non-chromatographic speciation analysis of chromium in natural waters. Int J Environ Anal Chem 92:1262–1275
Rodriguez E, Santos C, Azevedo R, Moutinho-Pereir J, Correia C, Dias MC (2012) Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol Biochem 53:94–100
Saygi KO, Tuzen M, Soylak M, Elci L (2008) Chromium speciation by solid phase extraction on Dowex M 4195 chelating resin and determination by atomic absorption spectrometry. J hazard Mater 153:1009–1014
Shah F, Kazi TG, Afridi HI, Khan AR, Arain SS, Arain MS, Panhwar AH (2016) Switchable dispersive liquid–liquid microextraction for lead enrichment: a green alternative to classical extraction techniques. Anal Methods 8:904–911
Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system. A review. Chemosphere 178:513–533
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Sharma N, Tiwari S, Saxena R (2016) On-line solid phase extraction method based on flow injection-FAAS using 1, 10-phenanthroline modified chelating resin for chromium speciation in industrial water samples. RSC Adv 6:10775–10782
Sogor C, Gaspar A, Posta J (1998) Flame atomic absorption spectrometric determination of total chromium and Cr(VI) in cigarette ash and smoke using flow injection/hydraulic high-pressure sample introduction. Microchem J 58:251–255
Sun HW, Kang WJ, Ha J, Liang SX, Shen SG (2004) Determination of Cr (III) and Cr (VI) in environmental waters by derivative flame atomic absorption spectrometry using flow injection on-line preconcentration with double-microcolumn adsorption. J Iran Chem Soc 1:40–46
Sun P, Liu ZT, Liu ZW (2009) Chemically modified chicken feather as sorbent for removing toxic chromium (VI) ions. Ind Eng Chem Res 48:6882–6889
Uluozlu OD, Tuzen M, Soylak M (2009) Speciation and separation of Cr (VI) and Cr (III) using coprecipitation with Ni2+/2-Nitroso-1-naphthol-4-sulfonic acid and determination by FAAS in water and food samples. Food Chem Toxicol 47:2601–2605
Vajpayee P, Sharma S, Tripathi R, Rai U, Yunus M (1999) Bioaccumulation of chromium and toxicity to photosynthetic pigments, nitrate reductase activity and protein content of Nelumbo nucifera gaertin. Chemosphere 39:2159–2169
Vanderveen JR, Durelle J, Jessop PG (2014) Design and evaluation of switchable-hydrophilicity solvents. Green Chem 16:1187–1197
Vernay P, Gauthier-Moussard C, Hitmi A (2007) Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 68:1563–1575
Wai CM, Tsay LM, Jim CY (1987) A two-step extraction method for differentiating chromium species in water. Microchim Acta 92:73–78
Xiashi Z, Zucheng J, Bin H, Mingfang L (2003) Cloud point extraction for speciation of chromium by electrothermal atomic absorption spectrometry. Chin J Anal Chem 31:1312–1316
Yamada T, Lukac PJ, George M, Weiss RG (2007) Reversible, room-temperature ionic liquids. Amidinium carbamates derived from amidines and aliphatic primary amines with carbon dioxide. Chem Mater 19:967–969
Zayed A, Lytle CM, Qian JH, Terry N (1998) Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 206:293–299
Funding
The funding for the project was approved by the Higher Education Commission, Project No. 20-2834/R&D/HEC/12 entitled “Nutritional Assessment of Selected Non-Traditional Vegetables of Sindh”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tasneem Gul Kazi declares that she no conflict of interest. Nusrat Shahab Memon declares that he no conflict of interest. Saghir Ahmed Shaikh declares that he no conflict of interest. Safia Sanam Memon declares that she no conflict of interest.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kazi, T.G., Memon, N.S., Shaikh, S.A. et al. Speciation and Separation of Trace Quantities of Hexavalent and Trivalent Chromium Species in Aqueous Extract of Wild Leafy Vegetables Using Multistep Pre-concentration Method. Food Anal. Methods 12, 1964–1972 (2019). https://doi.org/10.1007/s12161-019-01544-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-019-01544-1