Abstract
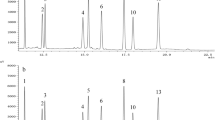
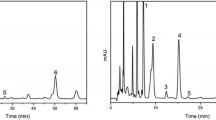
The fatty acid composition and the amounts of individual and total sterols in vegetable oils are the main analyses applied in the food industry to establish the oil nature. While the fatty acid composition is a relatively simple, fast analysis, the determination of phytosterols requires a laborious and time-consuming sample preparation. Both methods require a relatively large amount of oil, which may be an important drawback when only small samples are available. In this study, an analytical procedure that combines the sample preparation of both determinations is proposed to analyze small amounts of seed oils. From a single sample preparation, the total analysis time was considerably shortened. By applying a total methylation, the triacylglycerols and free fatty acids were transformed into fatty acid methyl ester (FAME) derivatives. Likewise, free sterols were completely released from their conjugated forms. Then, the derivatized oil was fractionated by solid-phase extraction into two fractions containing the FAME and free sterols, respectively. Both fractions were analyzed by gas-liquid chromatography. The analytical changes introduced provided reliable results for the main fatty acids and the major sterols in terms of accuracy and repeatability. Compared to the standard procedures, the time for sample preparation was reduced by half. In addition, it was much less laborious and required less volume of organic solvents, which reduced considerably the total cost of analysis and solvent waste. Consequently, the method proposed can be adopted as routine analysis in laboratories of oil quality control in the food industry.

Similar content being viewed by others
References
Abidi SL (2001) Chromatographic analysis of plant sterols in foods and vegetable oils. J Chromatogr A 935(1-2):173–201. https://doi.org/10.1016/S0021-9673(01)00946-3
Alberici RM, Fernandes GD, Porcari AM, Eberlin MN, Barrera-Arellano D, Fernández FM (2016) Rapid fingerprinting of sterols and related compounds in vegetable and animal oils and phytosterol enriched-margarines by transmission mode direct analysis in real time mass spectrometry. Food Chem 211:661–668. https://doi.org/10.1016/j.foodchem.2016.05.057
AOCS (1997) Method Ch 6-91 in official methods and recommended practices of the American Oil Chemists’ Society. AOCS Press, Champaign
Codex Alimentarius (2001) Codex standard for named vegetable oils. CODEX STAN 210–1999
EEC (1991) Commission regulation no. 2568/91. Determination of the composition and content of sterols by capillary-column gas chromatography. Official Journal of the European Communities. No. L 248/15–22
Fernández-Cuesta A, Aguirre-González M, Ruiz-Méndez MV, Velasco L (2012) Validation of a method for the analysis of phytosterols in sunflower seeds. EurJ Lipid Sci Technol 114(3):325–331. https://doi.org/10.1002/ejlt.201100138
Frankel EN (2012) Free radical oxidation. In: Frankel EN (ed) Lipid oxidation, 2nd edn. Woodhead publishing limited, Cambridge, pp 15–24
Garcés R, Mancha M (1993) One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal Biochem 211(1):139–143. https://doi.org/10.1006/abio.1993.1244
Gordon MH, Griffith RE (1992) Steryl ester analysis as an aid to the identification of oils in blends. Food Chem 43(1):71–78. https://doi.org/10.1016/0308-8146(92)90244-V
Hopia AI, Piironen VI, Koivistoinen PE, Hyvönen LE (1992) Analysis of lipid classes by solid-phase extraction and high-performance size-exclusion chromatography. J Am Oil Chem Soc 69:772–776. https://doi.org/10.1007/BF02635913
ISO (1991) Method 6799. Animal and vegetable fats and oils—determination of composition of the sterol fraction—method using gas chromatography. International Organization for Standardization
ISO (1999) Method 12228–2. Determination of individual and total sterols contents—gas chromatographic method. International Organization for Standardization
IUPAC (1992) Standard methods for the analysis of oils, fats and derivatives, 7thedn. International union of pure and applied chemistry. Blackwell Scientific, Oxford
Kumar S, Mawlong I, Singh D (2016) Phytosterol recovery from oilseeds: recent advances. J Food Process Eng 40:e12466. https://doi.org/10.1111/jfpe.12466
Lagarda MJ, García-Llatas G, Farré R (2006) Analysis of phytosterols in foods. J Pharm Biomed Anal 41(5):1486–1496. https://doi.org/10.1016/j.jpba.2006.02.052
Magnusson B, Örnemark U (2014) Eurachem guide: the fitness for purpose of analytical methods—a laboratory guide to method validation and related topics. Available: http://www.eurachem.org [Accessed June 24, 2015]
Mathison B, Holstege D (2013) A rapid method to determine sterol, erythrodiol, and uvaol concentrations in olive oil. J Agric Food Chem 61(19):4506–4513. https://doi.org/10.1021/jf400254k
Moreau RA, Whitaker BD, Hicks KB (2002) Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog Lipid Res 41(6):457–500. https://doi.org/10.1016/S0163-7827(02)00006-1
Seçmeler Ö, GüçlüÜstündağ Ö (2017) A rapid in-house validated GC-FID method for simultaneous determination of lipophilic bioactives in olive oil: squalene, α-tocopherol, and β-sitosterol. Eur J Lipid Sci Technol 119(1):1500420. https://doi.org/10.1002/ejlt.201500420
Spencer GF, Herb SF, Gormisky PJ (1976) Fatty acid composition as a basis for identification of commercial fats and oils. J Am Oil Chem Soc 53(3):94–96. https://doi.org/10.1007/BF02635956
Tena N, Wang SC, Aparicio-Ruiz R, García-González DL, Aparicio R (2015) In-depth assessment of analytical methods for olive oil purity, safety, and quality characterization. J Agric Food Chem 63(18):4509–4526. https://doi.org/10.1021/jf5062265
Toivo J, Piironen V, Kalo P, Varo P (1998) Gas chromatographic determination of major sterols in edible oils and fats using solid-phase extraction in sample preparation. Chromatographia 48(11-12):745–750. https://doi.org/10.1007/BF02467642
Funding
This work was funded by “Junta de Andalucía” through project P12-AGR-4622 and the Spanish Ministry of Economy, Industry and Competitiveness through project AGL2013–45110-R. The authors thank to Irene Pérez de la Rosa for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Aída García-González declares that she has no conflict of interest. Joaquín Velasco declares that he has no conflict of interest. Leonardo Velasco declares that he has no conflict of interest. M. Victoria Ruiz-Méndez declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
García-González, A., Velasco, J., Velasco, L. et al. An Analytical Simplification for Faster Determination of Fatty Acid Composition and Phytosterols in Seed Oils. Food Anal. Methods 11, 1234–1242 (2018). https://doi.org/10.1007/s12161-017-1111-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-1111-z