Abstract
A reversed phase high-performance liquid chromatography method (HPLC) with coulometric electrochemical detection has been applied and validated for the simultaneous analysis of pyridoxamine (PM), pyridoxal (PL), and pyridoxine (PN) in cereal products. Isocratic separation was achieved using a C18 column with a mobile phase consisting of methanol-phosphate buffer (10:90) and 0.018 M trimethylamine adjusted to pH 3.55 with 85% orthophosphoric acid. The limits of detection for PM, PL, and PN were 0.28, 0.36, and 0.43 ng mL−1, respectively, with vitamins recoveries ranging from 90.4 to 98.1%. The applied method for the analysis of B6 vitamins naturally present in grain products, offers a simple and fast sample preparation without derivatization. To understand vitamer separation further, methods of computational chemistry were employed. Specifically, density functional theory (DFT) was used to determine electrostatic potentials, as well as the charges of each vitamer. A number of correlations were established between these properties and elution order.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The B6 vitamers (Fig. 1) are a group of water-soluble forms of vitamin B6, which are chemically quite distinct compounds. Vitamin B6 represents a group of highly bioavailable 3-hydroxy-2-methylpyridine derivatives that exist in food as phosphorylated and non-phosphorylated forms (Ball 2006; Eitenmiller et al. 2008). Vitamin B6 are also enzymatic co-factors required for over 140 biochemical reactions involved in amino acid biosynthesis and degradation, as well as sugar, and fatty acid metabolism (Ball 2006). Vitamin B6 is necessary in the synthesis of both serotonin and GABA neurotransmitters that control depression, pain perception, and anxiety. Most vitamin B6-dependent enzymes catalyze reactions including transamination, decarboxylation, dehydration, desulfhydration, racemization, cleavage, and synthesis, among other processes including the activation of glycine during the initial stages of heme production (Bui and Small 2012; Denslow et al. 2005; Sorolla et al., 2010). Interestingly, vitamin B6 has recently been found to be a potent antioxidant, with the particular ability to quench reactive oxygen species such as superoxide, and singlet oxygen (Tambasco-Studart et al. 2005). Recent studies have shown that DNA damage resulting from deficiencies of micronutrients such as vitamins B6, and B12 are likely to be major contributing factors to cancer, as vitamin B6 plays a crucial role in 1-carbon metabolism, which involve DNA synthesis, and methylation (Larsson et al. 2005; Liu et al. 2008).
Although all organisms are dependent on vitamin B6, only microorganisms and plants are able to synthesize it de novo. Mammals, including humans have a salvage pathway, lack the enzymes for the novo synthesis of B6 and rely on uptake of the vitamin from food, with plants as the most important source for human nutrition (Hellmann and Mooney 2010; di Salvo et al. 2012).
The B6 vitamers exist in various forms of pyridoxine (PN), pyridoxal (PL), and pyridoxamine (PM) (Fig. 1), which during metabolic conversion becomes phosphorylated at the 5-hydroxymethyl position by a kinase required as a co-factor in enzymatic reactions. In plants, PN can exist in quite significant amounts as a β-glucoside. Pyridoxine is the major vitamer in cereal products where it may also exist as the glucosylated adduct, 5′-O-(ß-D glucopyranosyl) pyridoxine, which is of limited bioavailability (Gregory and Kirk 1981). Animal products contain more bioavailable forms of vitamin B6 compared to plant food sources, which comprise approximately 50% of human bioavailability (Gregory 2012). The bioavailability of PN in plant-based foods varies considerably, ranging from 0 to 80% (Kabir et al. 1983).
Pyridoxal-5′-phosphate (PLP), on the other hand, has been reported to be the most biologically active form of vitamin B6 and functions as a co-factor crucial for numerous enzymatic reactions (di Salvo et al. 2012; Eitenmiller et al., 2008; van der Ham et al. 2012).
The high variability of B6 vitamins and their low concentrations in food products cause difficulties in analysis. Moreover, grains and grain products are food matrixes that show special analytical difficulties, as they are starchy products that contain several interfering compounds (Lebiedzińska et al. 2008). These reasons are why the majority of methods target determination of total vitamin B6 content in grain product analysis.
Traditionally, the Association of Official Analytical Chemists (AOAC 2003) recommends microbiological assays of B6, which are perceived as time-consuming. Moreover, chemical and microbial methods previously used failed to accurately measure the nutritionally active vitamin forms, mainly due to varying extraction efficiency and stability of the vitamers. The advantage of chromatographic methods is thus the possibility to characterize vitamer forms individually in foods (Eitenmiller et al. 2008; Gregory 2012). Therefore, new methods should be developed that allow this instead of the approach including conversion of vitamers to a single analyte forms.
A variety of LC methods are reported for the determination of vitamin B6 in pharmaceuticals (Jin et al. 2012) and in food products (Ciulu et al. 2011; Gliszczyńska-Świgło and Rybicka 2015; Langer and Lodge 2014; Santos et al. 2012). Most separation and detection procedures are reversed-phase high-performance liquid chromatography (HPLC) techniques (Eitenmiller et al. 2008) coupled with fluorescent detections (Bui and Small 2012; Gatti and Gioia 2005; Gliszczyńska-Świgło and Rybicka 2015; Langer and Lodge 2014), UV (Ciulu et al. 2011; Jin et al. 2012; Langer and Lodge 2014), and MS (Hälvin et al. 2013; van der Ham et al. 2012). Methods using electrochemical detection (ED) have also been proposed but applications to samples other than pharmaceuticals supplements and animal foods are few (Lebiedzińska et al. 2007; Marszałł et al. 2005).
Although several analytical methods have been developed for the determination of individual vitamin B6 compounds, only few may be applied to the analysis of grain samples. Thus, there is a need for reliable, accurate, and precise analytical methods for vitamins in cereal foods as it is essential for determining the dietary intake of vitamin B6. The aim of the present work is thus to determine B6 vitamers present in cereal products using HPLC coupled with ED detection. To further understand the elution profile and the nature of separation, computational chemistry, specifically density functional theory, was applied to predict the electrostatic properties of each vitamer under consideration and was positively correlated with retention times.
Experimental
Instrumentation
Chromatographic analyses of pyridoxamine (PM), pyridoxal (PL), and pyridoxine (PN) in cereal products were performed on an HPLC system that consisted of a pump P 580 (Dionex, USA) and a sample injector (Rheodyne) with a 20-μL loop, coupled with Coulochem II electrochemical detector (ESA, USA) and diode array UVD 340S (Dionex, USA). Chromatographic separation and quantification were achieved on a Chromeleon Chromatography Management System using Supelco (Germany) C18, 5 μm (250 mm × 4.6 mm).
Reagents and Solutions
All reagents were of analytical grade. Pyridoxine hydrochloride, pyridoxamine hydrochloride (purity ≥ 99.0%), and pyridoxal hydrochloride (purity ≥ 99.5%) were provided by Fluka (Biochemica). Water-soluble papain (from Carica papaya) and diastase (from fungi) were obtained from Merck (catalog no. 103604 and 107144, respectively); α-amylase (from Aspergillus oryzae) was purchased from Sigma-Aldrich (catalog no. A-6211). Trimethylamine hydrochloride and sodium phosphate dibasic heptahydrate were purchased from Sigma-Aldrich (St. Louis, MO, USA) while orthophosphoric acid (85% w/w) was obtained from Riedel-de Haën (Seelze, Germany). Methanol, HPLC grade, was supplied by Baker (HPLC Analyzed). Purified water was obtained from Compact Mili-Q system (Millipore, Milford, MA, USA). The phosphate buffer (pH 3.55) was prepared using 0.05 mol L−1 sodium phosphate dibasic heptahydrate and 85% orthophosphoric acid. Enzymes were dissolved in distilled water to a concentration of 100 mg mL−1. Vitamin standards were prepared by dissolving 0.010 g of each vitamin in 100 mL of ultrapure water to 0.100 mg mL−1. Solutions were stored separately in the dark at 4 °C. Standards were prepared daily by appropriate dilution of individual stocks from the mobile phase.
Samples and Reference Materials
The products analyzed in this study including wheat bread and wholemeal flour were randomly purchased from the local market in Gdansk (Poland). Firstly, they were homogenized and subsampled (50–100 g) and then stored at − 18 °C until analysis. The certified reference material wholemeal flour (CRM 121), which was obtained from the Community Bureau of Reference, BCR (Belgium), was also subsampled (5 g) and stored frozen.
Sample Preparation
Samples were first hydrolyzed with mineral acids and then enzymatic treatments were applied to effectively liberate the B6 vitamers analyzed from the sample matrix and to correctly determine their concentration. Phosphorylated vitamin esters were converted to free vitamin forms using the enzyme mixtures. The extraction procedure applied in this study was based on a combination of acid digestion and enzymatic hydrolysis, according to the study of Esteve et al. (1998) and recommended by the AOAC (2003).
Extraction Procedure
Samples of cereal products, i.e., bread, flour, and wholemeal flour (CRM 121) were weighed accurately (2.0 ± 0.0001 g) and left for 30 min in an autoclave at 121 °C with 50 mL of 0.1 M HCl previously added. Then, the sample was cooled and pH adjusted to 4.5 with 2.5 M sodium acetate buffer and mixture of papain (40 mg) and diastase (100 mg) per 1 g of sample was added. An enzyme blank was always used to correct for the addition of vitamins from the enzyme suspension. After enzyme incubation at 37 °C for 18 h, samples were heated to 100 °C for 5 min to inactivate the enzyme and diluted to 100 mL with distilled water. Prior to HPLC analysis, samples were filtered through a Whatman 1 Chr filter paper. All preparations were conducted under dim light, and samples were kept in the dark in an amber flask at + 4 °C (24 h), then at 18 °C until analysis.
Chromatographic Conditions
Isocratic separation of B6 vitamers was performed on an LC 18 column of 5 μm (25 cm × 4.6 mm) which was termostated at 25 °C. The mobile phase was filtered through a 0.22-μm membrane filter and vacuum degassed prior to use, consisting of methanol-phosphate buffer (10:90), and 0.018 M trimethylamine reagent adjusted to pH 3.55 with 85% orthophosphoric acid. The volume of injection was 20 μL.
Pyridoxamine, pyridoxal, and pirydoxine were detected using a Coulochem II (model 5020A, ESA, USA) electrochemical detector equipped with a dual analytical cell (model 5010), with guard cell (model 5020). The guard cell was connected in-line before the injection port and was used to eliminate the interference with baseline stability. The detector response was set to give a full-scale detection for a 1- and 50-μA current output received from the analytical cell. The HPLC was operated in a constant flow mode, and the flow rate was kept at 1 mL min−1. The peak area of the electrochemical signal at the porous graphite electrode was used for quantitative analysis.
Statistics and Visualization
Statistical analyses such as linear regression and tests for intercept, slope, and intra- and inter-day were conducted with the use of Statistica for Windows (version 10, Statsoft, 1984–2011, Cracow, Poland) software-package.
Computational Methodology
All quantum chemical calculations reported in this study were carried out in the gas phase using the B3LYP hybrid functional and the 6-311g(d) basis set as implemented in Gaussian 09 suite of programs for electronic structure calculations (Gaussian 09 n.d). The primary aim of these calculations was to determine the electrostatic potentials of PM, PL, and PN vitamers. Geometries of each vitamer were taken from the PubChem Compound Database, (CIDs: 1052, 1050, and 1054) and used for single-point energy calculations. In addition to electrostatic potentials for each vitamer, Mulliken and natural charges were also computed.
Results
Validation
In order to guarantee accurate, precise, and linear results, the B6 vitamer concentration ranges were adjusted to their levels in food. All calculations were based on the calibration graphs constructed as a function of vitamer standard concentrations (ng mL−1) vs. peak area. The calibration graphs were characterized with linearity and a high correlation coefficient (r ≥ 0.998). All parameters obtained for regression equations are given in Table 1. The CRM material (CRM 121) linearity was established within the concentration ranges of 1–50, 5.0–350, and 1.5–200 ng mL−1 for PM, PL, and PN, respectively.
The limits of detection (LOD) for pyridoxamine, pyridoxal, and pyridoxine were 0.28, 0.36, and 0.43 ng mL−1, respectively, with the detector sensitivity set at 1 μA. LODs were calculated within three standard deviations of background noise, with the standard finally diluted in the same buffer as the food sample, with a limit of quantification (LOQ) of 10 × SD. The LOQ values obtained for pyridoxamine, pyridoxal, and pyridoxine were 1.00, 1.20, and 1.50 ng mL−1, respectively (detector sensitivity 1 μA).
The 1-day repeatability was evaluated by 10-fold measuring the same wholemeal flour sample (CRM121), where intermediate precision was determined by analyzing the same sample during five consequent days. All validation results are reported in Table 1.
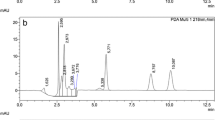
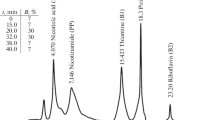
The accuracy of the method was checked using the certified material CRM 121 wholemeal flour, which was used to verify the accuracy of results and monitor method performance. The analytical results obtained for CRM 121 wholemeal flour were highly satisfactory, and the recovery (as a measure of accuracy) for the B6 vitamin amounted to 99% and RSD (as a measure of precision) equaled 1.88%. The exemplary chromatogram obtained for wholemeal flour is shown in Fig. 2a.
a Chromatogram of vitamin B in reference materials CRM 121 (wholemeal flour) 1—PM (t R 3.7 min, 0.08 μg mL-1), 2—PL (t R 4.7 min, 0.54 μg mL-1), 3—PN (t R 5.6 min, 0.12 μg mL-1), ED sensitivity 50 μA. b Chromatogram of vitamin B in wheat bread sample (A) 2—PL (t R 4.7 min, 4.4 ng mL−1), 3—PN (t R 5.6 min, 32.2 ng mL−1)
Quantitative Analysis of the B6 Vitamins in the Commercial Plant Food Products
The proposed analytical approach was verified by analysis of commercially available plant food products (Fig. 2b). Vitamin B6 concentration estimate was performed based on calibration curves obtained as a function of concentration (ng mL−1) vs. peak area for pyridoxamine, pyridoxal, and pyridoxine, Table 2.
Computational Modeling Using Density Functional Theory
To provide a better understanding of separation order based on chromatographic data (Fig. 2a, b), molecular properties were determined from single-point energy calculations. Figure 3 provides a graphical summary of electrostatic potential surfaces for PM, PL, and PN. Electrostatic potentials provide a simple, conceptual means to understand surface interactions with separation medium. Mulliken and natural charges also provide a complementary and quantitative understanding. Because all molecules interact with the same medium, it is expected that elution order should correlate to the electrostatic potential differences between functional groups.
As shown in Table 3, a direct relationship can be seen between the order of elution and the sum of electrostatic potentials, where lower potentials correlate to higher retention times. Mulliken and natural charges are also shown to increase as expected for PM, PL, and PN, respectively. Separation of B6 vitamer fragments is quite different from that previously reported for vitamin B12 derivatives (cobalamins). In contrast to B6 vitamers, cobalamins cannot be simply separated (Lebiedzińska et al. 2007). Based on DFT calculations, this may be attributed to the much larger size of B12 compared to B6, leading to an electrostatic potential distribution that is more disperse, where functional group modifications of the outer coordination sphere have relatively little effect on the electrostatic properties. Based on this analysis, it is expected that all cobalamins should interact in a similar way with the separation surface, while the B6 vitamers will produce notable electrostatic changes with even a small modification of the structure, due to their smaller size.
Discussion
Comprehensive vitamin B6 analysis is required for food analysis, although differentiation between unphosphorylated vitamers and the 5-phosphoric acid esters is usually not. The principle method of extraction is release of B6 vitamins, their glucosated forms from proteins, and conversion of the phosphorylated compounds to pyridoxine, pirydoxamine, and pirydoxal (Lebiedzińska et al. 2008). The critical part of the analysis is the enzymatic treatment necessary to liberate protein- and glucosated phosphate-bound vitamins (Lebiedzińska et al. 2008). Recent research (Ball 2006; Gregory 2012) has shown that PN-glycoside has a much lower bioavailability than pyridoxine, and it is therefore reasonable to assay all B6 vitamers separately.
Sample extractions with mineral acids or enzymatic digestions of food matrices are analytical procedures most frequently used before quantitative measurement of vitamin B6 vitamers (Eitenmiller et al. 2008). Glucosidase side activity of enzyme preparations such as diastase is high enough to hydrolyze glucosidic linkages in bound vitamin forms. A microbiological procedure, which involves an acid hydrolysis and enzymatic steps during sample extraction, is used in most reports (Sampson et al. 1995; Sampson et al. 1996). However, in this procedure, any PN-glucoside (PNG) in the sample is converted into PN (Gregory 2012). Consequently, much of the literature may report PNG present in plant foods as PN. Plant foods contain substantial fractions of vitamin B6 as PN-glucoside, and thus, such methods may lead to over estimation of nutritional content since the resulting data does not reflect the B6 content, or the partial bioavailability of PN-glucoside. Most vitamin B extraction procedures use HCl hydrolysis because vitamins are stable in acidic environments and break protein complexes, effectively liberating vitamins from cellular material in most sample matrices.
However, Ollilainen et al. (2001) has shown that vitamin B6 compounds may be measured in their intact forms, and the original distribution of vitamers can be preserved during the analytical procedure, which included cold perchloric acid extraction followed by enzymatic digestions before the liquid chromatographic analysis. Moreover, Gatti and Gioia (2005) have developed an HPLC method with fluorescence detection for simultaneous analysis of B6 vitamers and B2 in commercial vitaminized milk and human breast milk. Other methods of detection of vitamin B6 are based on redox reactions with electrochemical detectors, such as amperometric and coulometric. Early assays followed the oxidation of PN at a carbon paste electrode. Flow injection and sequential injection techniques as well as the availability of modified glassy carbon electrodes have also added speed and improved selectivity to electrochemical assays for vitamin B6 (Desai et al. 2008; Eitenmiller and Landen 1999; Eitenmiller et al. 2008).
Nishimura et al. (2008) have established a method for the determination of individual vitamin B6 compounds on the basis that the B6 vitamers are converted by enzymes into highly fluorescent 4-pyridoxolactone. HCl hydrolysis is also used to convert the phosphoester forms into their corresponding free forms. Seven vitamin B6 compounds were determined in animal, fish, and vegetable food using the enzymatic-based HPLC method developed by Do et al. (2012).
In our extraction process, the 5′phosphate forms, as well as pyridoxine-β-glucoside were hydrolyzed with HCl and determined as their free forms in a few minutes, using data from computational chemistry analysis. Separation of B6 vitamers is quite different from previously reported for vitamin B12 derivatives (cobalamins) separations. In contrast to B6, cobalamins cannot be simply separated (Lebiedzińska et al. 2007). As mentioned, based on DFT calculations, this may be attributed to the much larger size of B12 in comparison to B6. While the larger coordination sphere of the cobalamins results in an essentially uniform interaction with the separation medium, greater differences of electrostatic potential distribution of the B6 vitamers result in an elution profile that is well separated, due to their smaller size.
Coulometric detection enabled us determination of B6 free forms in a few minutes and provided higher sensitivity and selectivity than UV detection. It was due to the lack of interferences with other substances and the ability of ED to resolve peaks according to their voltammetric properties. The UV and ED detectors as well as FL one are rather inexpensive, but ED and FL characterize with clean blank and sample backgrounds and very high sensitivities.
Conclusions
The growing interest in interactions between diet and health requires a more precise analytical methodology for determination of vitamins in food products, which is essential for the calculation of dietary intake of vitamin B6 from food. Thus, the outlined method of B6 vitamer analysis is important to consumers as well as the scientific community. The presented method characterized with the satisfying sensitivity and selectivity and low limits of detection.
Finally, a unique aspect of this study is the combined theoretical approach used to determine elution order based on analysis of computationally predicted electrostatic potentials. Density functional theory was implemented to determine the charge characters of each fragment, and it was concluded that due to the relatively small size of B6, lower electrostatic potentials, and consequently higher atomic charges, correlate to longer retention times. According to our knowledge, this is the first report using computational chemistry combined with HPLC-ED. Our research has confirmed that grain products can constitute an important source of all three vitamers of B6 vitamin, including pyridoxine hydrochloride, and that vitamer concentrations can be routinely estimated using this method.
References
AOAC (2003) Official Methods of Analysis of AOAC (Association of Official Analytical Chemists) International (18th ed.). AOAC International, Arlington, VA, USA
Ball GMF (2006) Vitamins in foods. Analysis, bioavailability and stability. CRC, Press, Boca Raton
Bui LTT, Small DM (2012) The stability of pyridoxine hydrochloride used as a fortificant in Asian wheat flour noodles. Food Chem 130(4):841–846
Ciulu M, Solinas S, Floris I, Panzanelli A, Pilo MI, Piu PC, Spano N, Sanna G (2011) RP-HPLC determination of water-soluble vitamins in honey. Talanta 83:925–929
Denslow SA, Walls AA, Daub ME (2005) Regulation of biosynthetic genes and antioxidant properties of vitamin B6 vitamers during plant defense responses. Physiol Mol Plant Pathol 66:244–255
Desai PB, Kotkar RM, Srivastava AK (2008) Electrochemical behavior of pyridoxine hydrochloride (vitamin B6) at carbon paste electrode modified with crown ethers. J Solid State Electrochem 12(9):1067–1075
Do HTV, Ide Y, Mugo AN, Yagi T (2012) All-enzymatic HPLC method for determination of individual and total contents of vitamin B6 in foods. Food Nutr Res 56:5409. https://doi.org/10.3402/fnr.v56i0.5409
Eitenmiller RR, Landen WO Jr (1999) Vitamin analysis for the heath and food sciences. CRC Press, Boca Raton
Eitenmiller RR, Ye L, Landen WO Jr (2008) Vitamin analysis for the heath and food sciences. CRC Press Taylor and Francis Group, Boca Raton
Esteve MJ, Farré R, Frígola A, García-Cantabella JM (1998) Determination of vitamin B6 (pyridoxamine, pyridoxal and pyridoxine) in pork meat and pork meat products by liquid chromatography. J Chromatogr A 795:383–387
Gatti R, Gioia MG (2005) Liquid chromatographic determination with fluorescence detection of B6 vitamers and riboflavin in milk and pharmaceuticals. Anal Chim Acta 538:35–41
Gaussian 09. M. J. G. Frisch, W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. G. Mennucci, A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. E. M. Hada, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox
Gliszczyńska-Świgło A, Rybicka I (2015) Simultaneous determination of caffeine and water-soluble vitamins in energy drinks by HPLC with photodiode array and fluorescence detection. Food Anal Methods 8:139–146
Gregory JF (2012) Accounting for differences in the bioactivity and bioavailability of vitamers. Food Nutr Res 56:5809. https://doi.org/10.3402/fnr.v56i0.5809
Gregory JF, Kirk JR (1981) The bioavailability of vitamin B6 in foods. Nutr Rev 39:1–8
Hälvin K, Paalme T, Nisamedtinov I (2013) Comparison of different extraction methods for simultaneous determination of B complex vitamins in nutritional yeast using LC/MS-TOF and stable isotope dilution assay. Anal Bioanal Chem 405:1213–1222
van der Ham M, Albersen M, de Koning TJ, Visser G, Middendorp A, Bosma M, Verhoeven-Duif NM, de Sain-van der Velden MGM (2012) Quantification of vitamin B6 vitamers in human cerebrospinal fluid by ultra performance liquid chromatography-tandem mass spectrometry. Anal Chim Acta 712:108–114
Hellmann H, Mooney S (2010) Vitamin B6: A Molecule for Human Health. Molecules 15:442–459
Jin P, Xia L, Li Z, Che N, Zou D, Hu X (2012) Rapid determination of thiamine, riboflavin, niacin amide, pantothenic acid, pyridoxine, folic acid and ascorbic acid in vitamins and minerals tablets by high-performance liquid chromatography with diode array detector. J Pharm Biomed Anal 70:151–157
Kabir H, Leklem JE, Miller LT (1983) Relationship of the glycosylated vitamin B6 content of foods to vitamin B6 bioavailability in humans. Nutr Rep Int 28:709–716
Langer S, Lodge JK (2014) Determination of selected water-soluble vitamins using hydrophilic chromatography: a comparison of phodiode array, fluorescence and coulometric detection, and validation in a breakfast cereal matrix. J Chromatogr B 960:73–81
Larsson SC, Giovannucci E, Wolk A (2005) Vitamin B6 intake, alcohol consumption, and colorectal cancer: a longitudinal population-based cohort of women. Gastroenterology 128(7):1830–1837
Lebiedzińska A, Marszałł M, Kuta J, Szefer P (2007) Reversed-phase high-performance liquid chromatography method with coulometric electrochemical and ultraviolet detection for the quantification of vitamins B1 (thiamine), B6 (pyridoxamine, pyridoxal and pyridoxine) and B12 in animal and plant foods. J Chromatogr A 1173:71–80
Lebiedzińska A, Dąbrowska M, Szefer P, Marszałł M (2008) High-performance liquid chromatography method for the determination of folic acid in fortified food products. Toxicol Mech Methods 18:463–467
Liu S-Q, Cao M-L, Dong S-L (2008) Electrochemical and ultraviolet-visible spectroscopic studies on the interaction of deoxyribonucleic acid with vitamin B6. Bioelectrochemistry 74(1):164–169
Marszałł M, Lebiedzińska A, Czarnowski W, Szefer P (2005) High-performance liquid chromatography method for the simultaneous determination of thiamine hydrochloride, pyridoxine hydrochloride and cyanocobalamin in pharmaceutical formulations using coulometric electrochemical and ultraviolet detection. J Chromatogr A 1094:91–98
Nishimura S, Nagano S, Cral CA, Yokochi N, Yoshikane Y, Ge F, Yagi T (2008) Determination of individual vitamin B6 compounds based on enzymatic conversion to 4-pyridoxolactone. J Nutr Sci Vitaminol 54:18–24
Ollilainen V, Finglas PM, van den Berg H, de Froidmont-Görtz I (2001) Certification of B-group vitamins (B1, B2, B6 and B12) in four food reference materials. J Agric Food Chem 49:315–321
di Salvo ML, Budisa N, Contestabile R (2012) PLP-dependent enzymes: a powerful tool for metabolic synthesis of non-canonical amino acids http://www.beilstein-institut.de/Bozen2012/Proceedings/Budisa/Budisa.pdf
Sampson DA, Eoff LA, Yan XL, Lorenz K (1995) Analysis of free and glycosylated vitamin B6 in wheat by high-performance liquid chromatography. Cereal Chem 72(2):217–221
Sampson DA, Wen Q-B, Lorenz K (1996) Vitamin B6 and pyridoxine glucoside content of wheat and wheat flours. Cereal Chem 73(6):770–774
Santos J, Mendiola JA, Oliveira MBPP, Ibáňez Herrero M (2012) Sequential determination of fat- and water-soluble vitamins in green leafy vegetables during storage. J Chromatogr A 1261:179–188
Sorolla MA, Rodríguez-Colman MJ, Tamarit J, Ortega Z, Lucas JJ, Ferrer I, Ros J, Cabiscol E (2010) Protein oxidation in Huntington disease affects energy production and vitamin B6 metabolism. Free Radic Biol Med 49:612–621
Tambasco-Studart M, Titiz O, Raschle T, Forster G, Amrhein N, Fitzpatrick TB (2005) Vitamin B6 biosynthesis in higher plants. Proc Natl Acad Sci U S A 102(38):13687–13692
Funding
Visiting professorship of Pawel M. Kozlowski at the Medical University of Gdansk was partially supported by the Ministry of Science and Higher Education of the Republic of Poland, from the quality-promoting subsidy under the Leading National Research Centre (KNOW) program for the years 2012-2017.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This article does not contain any studies with human or animal subjects.
Conflict of Interest
Anna Lebiedzińska declares that she has no conflict of interest. Marcin L. Marszałł declares that he has no conflict of interest. Małgorzata Grembecka declares that she has no conflict of interest. Jakub Czaja declares that he has no conflict of interest. Piotr Szefer declares that he has no conflict of interest. Jadwiga Kuta declares that she has no conflict of interest. Brady D. Garabato declares that he has no conflict of interest. Pawel M. Kozlowski declares that he has no conflict of interest.
Informed Consent
Not applicable.
Additional information
Pawel M. Kozlowski is a visiting professor at the Department of Food Sciences, Medical University of Gdansk.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lebiedzińska, A., Marszałł, M.L., Grembecka, M. et al. Detection of B6 Vitamers in Grain Products: Experimental and Computational Studies. Food Anal. Methods 11, 725–732 (2018). https://doi.org/10.1007/s12161-017-1045-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-1045-5